Page 39 - Read Online
P. 39
Gasparello et al. J Cancer Metastasis Treat 2019;5:52 I http://dx.doi.org/10.20517/2394-4722.2019.17 Page 7 of 11
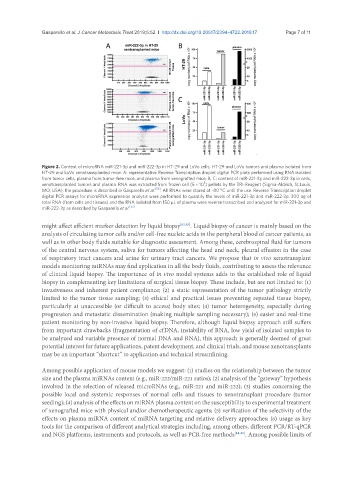
Figure 2. Content of microRNA miR-221-3p and miR-222-3p in HT-29 and LoVo cells, HT-29 and LoVo tumors and plasma isolated from
HT-29 and LoVo xenotransplanted mice. A: representative Reverse Transcription droplet digital PCR plots performed using RNA isolated
from tumor cells, plasma from tumor-free mice, and plasma from xenografted mice; B, C: content of miR-221-3p and miR-222-3p in cells,
5
xenotransplanted tumors and plasma. RNA was extracted from frozen cell (5 × 10 ) pellets by the TRI-Reagent (Sigma-Aldrich, St.Louis,
MO, USA), the procedure is described in Gasparello et al. [55] . All RNAs were stored at -80 °C until the use. Reverse Transcription droplet
digital PCR assays for microRNA expression analysis were performed to quantify the levels of miR-221-3p and miR-222-3p. 300 ng of
total RNA (from cells and tissues) and the RNA isolated from 150 µL of plasma were reverse transcribed and analyzed for miR-221-3p and
miR-222-3p as described by Gasparello et al. [55]
might affect efficient marker detection by liquid biopsy [82,83] . Liquid biopsy of cancer is mainly based on the
analysis of circulating tumor cells and/or cell-free nucleic acids in the peripheral blood of cancer patients, as
well as in other body fluids suitable for diagnostic assessment. Among these, cerebrospinal fluid for tumors
of the central nervous system, saliva for tumors affecting the head and neck, pleural effusion in the case
of respiratory tract cancers and urine for urinary tract cancers. We propose that in vivo xenotransplant
models monitoring miRNAs may find application in all the body fluids, contributing to assess the relevance
of clinical liquid biopsy. The importance of in vivo model systems adds to the established role of liquid
biopsy in complementing key limitations of surgical tissue biopsy. These include, but are not limited to: (1)
invasiveness and inherent patient compliance; (2) a static representation of the tumor pathology strictly
limited to the tumor tissue sampling; (3) ethical and practical issues preventing repeated tissue biopsy,
particularly at unaccessible (or difficult to access) body sites; (4) tumor heterogeneity, especially during
progression and metastatic dissemination (making multiple sampling necessary); (5) easier and real-time
patient monitoring by non-invasive liquid biopsy. Therefore, although liquid biopsy approach still suffers
from important drawbacks (fragmentation of cfDNA, instability of RNA, low yield of isolated samples to
be analyzed and variable presence of normal DNA and RNA), this approach is generally deemed of great
potential interest for future applications, patent development, and clinical trials, and mouse xenotransplants
may be an important “shortcut” to application and technical streamlining.
Among possible application of mouse models we suggest: (1) studies on the relationship between the tumor
size and the plasma miRNAs content (e.g., miR-222/miR-221 ratios); (2) analysis of the “gateway” hypothesis
involved in the selection of released microRNAs (e.g., miR-221 and miR-222); (3) studies concerning the
possible local and systemic responses of normal cells and tissues to xenotransplant procedure (tumor
seeding); (4) analysis of the effects on miRNA plasma content on the susceptibility to experimental treatment
of xenografted mice with physical and/or chemotherapeutic agents; (5) verification of the selectivity of the
effects on plasma miRNA content of miRNA targeting and relative delivery approaches; (6) usage as key
tools for the comparison of different analytical strategies including, among others, different PCR/RT-qPCR
and NGS platforms, instruments and protocols, as well as PCR-free methods [84-86] . Among possible limits of