Page 197 - Read Online
P. 197
Borniger. J Cancer Metastasis Treat 2019;5:23 I http://dx.doi.org/10.20517/2394-4722.2018.107 Page 7 of 18
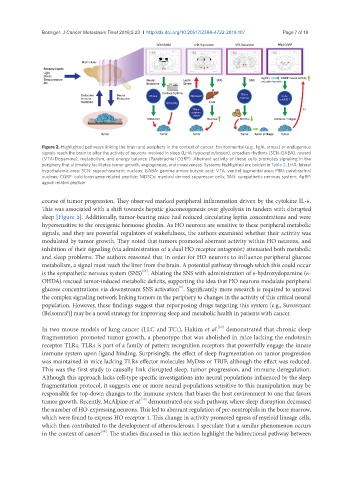
Figure 2. Highlighted pathways linking the brain and periphery in the context of cancer. Environmental (e.g., light, stress) or endogenous
signals reach the brain to alter the activity of neurons involved in sleep (LHA hypocretin/orexin), circadian rhythms (SCN-GABA), reward
(VTA-Dopamine), metabolism, and energy balance (Parabrachial CGRP). Aberrant activity of these cells promotes signaling in the
periphery that ultimately facilitates tumor growth, angiogenesis, and invasiveness. Systems highlighted are bolded in Table 2. LHA: lateral
hypothalamic area; SCN: suprachiasmatic nucleus; GABA: gamma amino butyric acid; VTA: ventral tegmental area; PBN: parabrachial
nucleus; CGRP: calcitonin gene related peptide; MDSCs: myeloid derived suppressor cells; SNS: sympathetic nervous system; AgRP:
agouti related peptide
course of tumor progression. They observed marked peripheral inflammation driven by the cytokine IL-6.
This was associated with a shift towards hepatic gluconeogenesis over glycolysis in tandem with disrupted
sleep [Figure 2]. Additionally, tumor-bearing mice had reduced circulating leptin concentrations and were
hypersensitive to the orexigenic hormone ghrelin. As HO neurons are sensitive to these peripheral metabolic
signals, and they are powerful regulators of wakefulness, the authors examined whether their activity was
modulated by tumor growth. They noted that tumors promoted aberrant activity within HO neurons, and
inhibition of their signaling (via administration of a dual HO receptor antagonist) attenuated both metabolic
and sleep problems. The authors reasoned that in order for HO neurons to influence peripheral glucose
metabolism, a signal must reach the liver from the brain. A potential pathway through which this could occur
[35]
is the sympathetic nervous system (SNS) . Ablating the SNS with administration of 6-hydroxydopamine (6-
OHDA) rescued tumor-induced metabolic deficits, supporting the idea that HO neurons modulate peripheral
[6]
glucose concentrations via downstream SNS activation . Significantly more research is required to unravel
the complex signaling network linking tumors in the periphery to changes in the activity of this critical neural
population. However, these findings suggest that repurposing drugs targeting this system [e.g., Suvorexant
(Belsomra®)] may be a novel strategy for improving sleep and metabolic health in patients with cancer.
[13]
In two mouse models of lung cancer (LLC and TC1), Hakim et al. demonstrated that chronic sleep
fragmentation promoted tumor growth, a phenotype that was abolished in mice lacking the endotoxin
receptor TLR4. TLR4 is part of a family of pattern recognition receptors that powerfully engage the innate
immune system upon ligand binding. Surprisingly, the effect of sleep fragmentation on tumor progression
was maintained in mice lacking TLR4 effector molecules MyD88 or TRIF, although the effect was reduced.
This was the first study to causally link disrupted sleep, tumor progression, and immune deregulation.
Although this approach lacks cell-type specific investigations into neural populations influenced by the sleep
fragmentation protocol, it suggests one or more neural populations sensitive to this manipulation may be
responsible for top-down changes to the immune system that biases the host environment to one that favors
[49]
tumor growth. Recently, McAlpine et al. demonstrated one such pathway, where sleep disruption decreased
the number of HO-expressing neurons. This led to aberrant regulation of pre-neutrophils in the bone marrow,
which were found to express HO receptor 1. This change in activity promoted egress of myeloid lineage cells,
which then contributed to the development of atherosclerosis. I speculate that a similar phenomenon occurs
[49]
in the context of cancer . The studies discussed in this section highlight the bidirectional pathway between