Page 25 - Read Online
P. 25
Kondapuram et al. J Cancer Metastasis Treat 2019;5:32 I http://dx.doi.org/10.20517/2394-4722.2018.105 Page 3 of 25
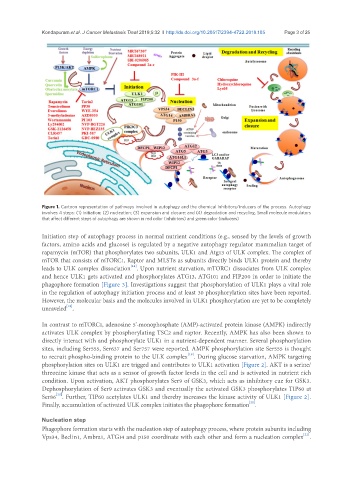
Figure 1. Cartoon representation of pathways involved in autophagy and the chemical inhibitors/inducers of the process. Autophagy
involves 4 steps: (1) initiation; (2) nucleation; (3) expansion and closure; and (4) degradation and recycling. Small molecule modulators
that affect different steps of autophagy are shown in red color (inhibitors) and green color (inducers)
Initiation step of autophagy process in normal nutrient conditions (e.g., sensed by the levels of growth
factors, amino acids and glucose) is regulated by a negative autophagy regulator mammalian target of
rapamycin (mTOR) that phosphorylates two subunits, ULK1 and Atg13 of ULK complex. The complex of
mTOR that consists of mTORC1, Raptor and MLST8 as subunits directly binds ULK1 protein and thereby
[18]
leads to ULK complex dissociation . Upon nutrient starvation, mTORC1 dissociates from ULK complex
and hence ULK1 gets activated and phosphorylates ATG13, ATG101 and FIP200 in order to initiate the
phagophore formation [Figure 3]. Investigations suggest that phosphorylation of ULK1 plays a vital role
in the regulation of autophagy initiation process and at least 30 phosphorylation sites have been reported.
However, the molecular basis and the molecules involved in ULK1 phosphorylation are yet to be completely
[18]
unraveled .
In contrast to mTORC1, adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) indirectly
activates ULK complex by phosphorylating TSC2 and raptor. Recently, AMPK has also been shown to
directly interact with and phosphorylate ULK1 in a nutrient-dependent manner. Several phosphorylation
sites, including Ser555, Ser637 and Ser757 were reported. AMPK phosphorylation site Ser555 is thought
[19]
to recruit phospho-binding protein to the ULK complex . During glucose starvation, AMPK targeting
phosphorylation sites on ULK1 are trigged and contributes to ULK1 activation [Figure 2]. AKT is a serine/
threonine kinase that acts as a sensor of growth factor levels in the cell and is activated in nutrient rich
condition. Upon activation, AKT phosphorylates Ser9 of GSK3, which acts as inhibitory cue for GSK3.
Dephosphorylation of Ser9 activates GSK3 and eventually the activated GSK3 phosphorylates TIP60 at
[20]
Ser86 . Further, TIP60 acetylates ULK1 and thereby increases the kinase activity of ULK1 [Figure 2].
[20]
Finally, accumulation of activated ULK complex initiates the phagophore formation .
Nucleation step
Phagophore formation starts with the nucleation step of autophagy process, where protein subunits including
[21]
Vps34, Beclin1, Ambra1, ATG14 and p150 coordinate with each other and form a nucleation complex .