Page 90 - Read Online
P. 90
Ida et al. J Cancer Metastasis Treat 2018;4:22 I http://dx.doi.org/10.20517/2394-4722.2017.75 Page 5 of 9
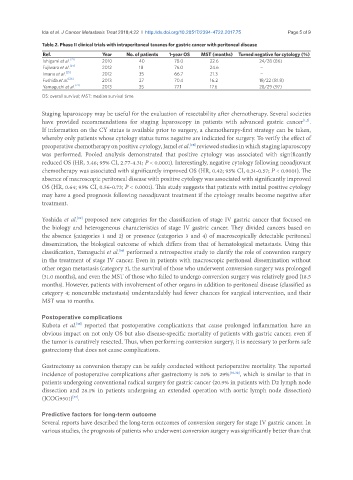
Table 2. Phase II clinical trials with intraperitoneal taxanes for gastric cancer with peritoneal disease
Ref. Year No. of patients 1-year OS MST (months) Turned negative for cytology (%)
[21]
Ishigami et al. 2010 40 78.0 22.6 24/28 (86)
Fujiwara et al. [24] 2012 18 76.0 24.6 -
Imano et al. [25] 2012 35 66.7 21.3 -
Fushida et al. [26] 2013 27 70.4 16.2 18/22 (81.8)
Yamaguchi et al. [27] 2013 35 77.1 17.6 28/29 (97)
OS: overall survival; MST: median survival time
Staging laparoscopy may be useful for the evaluation of resectability after chemotherapy. Several societies
have provided recommendations for staging laparoscopy in patients with advanced gastric cancer .
[1,2]
If information on the CY status is available prior to surgery, a chemotherapy-first strategy can be taken,
whereby only patients whose cytology status turns negative are indicated for surgery. To verify the effect of
preoperative chemotherapy on positive cytology, Jamel et al. reviewed studies in which staging laparoscopy
[28]
was performed. Pooled analysis demonstrated that positive cytology was associated with significantly
reduced OS (HR, 3.46; 95% CI, 2.77-4.31; P < 0.0001). Interestingly, negative cytology following neoadjuvant
chemotherapy was associated with significantly improved OS (HR, 0.42; 95% CI, 0.31-0.57; P < 0.0001). The
absence of macroscopic peritoneal disease with positive cytology was associated with significantly improved
OS (HR, 0.64; 95% CI, 0.56-0.73; P < 0.0001). This study suggests that patients with initial positive cytology
may have a good prognosis following neoadjuvant treatment if the cytology results become negative after
treatment.
Yoshida et al. proposed new categories for the classification of stage IV gastric cancer that focused on
[29]
the biology and heterogeneous characteristics of stage IV gastric cancer. They divided cancers based on
the absence (categories 1 and 2) or presence (categories 3 and 4) of macroscopically detectable peritoneal
dissemination, the biological outcome of which differs from that of hematological metastasis. Using this
classification, Yamaguchi et al. performed a retrospective study to clarify the role of conversion surgery
[30]
in the treatment of stage IV cancer. Even in patients with macroscopic peritoneal dissemination without
other organ metastasis (category 3), the survival of those who underwent conversion surgery was prolonged
(31.0 months), and even the MST of those who failed to undergo conversion surgery was relatively good (18.5
months). However, patients with involvement of other organs in addition to peritoneal disease (classified as
category 4; noncurable metastasis) understandably had fewer chances for surgical intervention, and their
MST was 10 months.
Postoperative complications
Kubota et al. reported that postoperative complications that cause prolonged inflammation have an
[31]
obvious impact on not only OS but also disease-specific mortality of patients with gastric cancer, even if
the tumor is curatively resected. Thus, when performing conversion surgery, it is necessary to perform safe
gastrectomy that does not cause complications.
Gastrectomy as conversion therapy can be safely conducted without perioperative mortality. The reported
incidence of postoperative complications after gastrectomy is 24% to 29% [30,32] , which is similar to that in
patients undergoing conventional radical surgery for gastric cancer (20.9% in patients with D2 lymph node
dissection and 28.1% in patients undergoing an extended operation with aortic lymph node dissection)
(JCOG9501) .
[33]
Predictive factors for long-term outcome
Several reports have described the long-term outcomes of conversion surgery for stage IV gastric cancer. In
various studies, the prognosis of patients who underwent conversion surgery was significantly better than that