Page 89 - Read Online
P. 89
Page 4 of 9 Ida et al. J Cancer Metastasis Treat 2018;4:22 I http://dx.doi.org/10.20517/2394-4722.2017.75
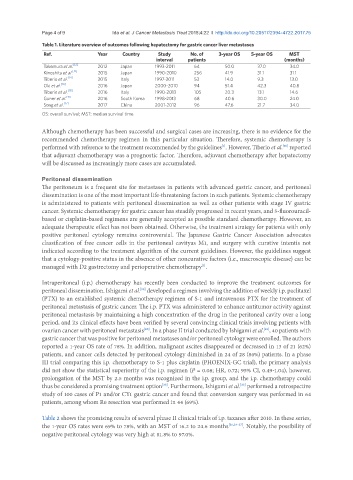
Table 1. Literature overview of outcomes following hepatectomy for gastric cancer liver metastases
Ref. Year Country Study No. of 3-year OS 5-year OS MST
interval patients (months)
Takemura et al. [12] 2012 Japan 1993-2011 64 50.0 37.0 34.0
[13]
Kinoshita et al. 2015 Japan 1990-2010 256 41.9 31.1 31.1
Tiberio et al. [14] 2015 Italy 1997-2011 53 14.0 9.3 13.0
Oki et al. [16] 2016 Japan 2000-2010 94 51.4 42.3 40.8
Tiberio et al. [18] 2016 Italy 1990-2013 105 20.3 13.1 14.6
[15]
Guner et al. 2016 South Korea 1998-2013 68 40.6 30.0 24.0
[17]
Song et al. 2017 China 2001-2012 96 47.6 21.7 34.0
OS: overall survival; MST: median survival time
Although chemotherapy has been successful and surgical cases are increasing, there is no evidence for the
recommended chemotherapy regimen in this particular situation. Therefore, systemic chemotherapy is
performed with reference to the treatment recommended by the guidelines . However, Tiberio et al. reported
[18]
[1]
that adjuvant chemotherapy was a prognostic factor. Therefore, adjuvant chemotherapy after hepatectomy
will be discussed as increasingly more cases are accumulated.
Peritoneal dissemination
The peritoneum is a frequent site for metastases in patients with advanced gastric cancer, and peritoneal
dissemination is one of the most important life-threatening factors in such patients. Systemic chemotherapy
is administered to patients with peritoneal dissemination as well as other patients with stage IV gastric
cancer. Systemic chemotherapy for gastric cancer has steadily progressed in recent years, and 5-fluorouracil-
based or cisplatin-based regimens are generally accepted as possible standard chemotherapy. However, an
adequate therapeutic effect has not been obtained. Otherwise, the treatment strategy for patients with only
positive peritoneal cytology remains controversial. The Japanese Gastric Cancer Association advocates
classification of free cancer cells in the peritoneal cavityas M1, and surgery with curative intentis not
indicated according to the treatment algorithm of the current guidelines. However, the guidelines suggest
that a cytology-positive status in the absence of other noncurative factors (i.e., macroscopic disease) can be
managed with D2 gastrectomy and perioperative chemotherapy .
[1]
Intraperitoneal (i.p.) chemotherapy has recently been conducted to improve the treatment outcomes for
peritoneal dissemination. Ishigami et al. developed a regimen involving the addition of weekly i.p. paclitaxel
[19]
(PTX) to an established systemic chemotherapy regimen of S-1 and intravenous PTX for the treatment of
peritoneal metastasis of gastric cancer. The i.p. PTX was administered to enhance antitumor activity against
peritoneal metastasis by maintaining a high concentration of the drug in the peritoneal cavity over a long
period, and its clinical effects have been verified by several convincing clinical trials involving patients with
ovarian cancer with peritoneal metastasis . In a phase II trial conducted by Ishigami et al. , 40 patients with
[20]
[21]
gastric cancer that was positive for peritoneal metastases and/or peritoneal cytology were enrolled. The authors
reported a 1-year OS rate of 78%. In addition, malignant ascites disappeared or decreased in 13 of 21 (62%)
patients, and cancer cells detected by peritoneal cytology diminished in 24 of 28 (86%) patients. In a phase
III trial comparing this i.p. chemotherapy to S-1 plus cisplatin (PHOENIX-GC trial), the primary analysis
did not show the statistical superiority of the i.p. regimen (P = 0.08; HR, 0.72; 95% CI, 0.49-1.04), however,
prolongation of the MST by 2.5 months was recognized in the i.p. group, and the i.p. chemotherapy could
thus be considered a promising treatment option . Furthermore, Ishigami et al. performed a retrospective
[23]
[22]
study of 100 cases of P1 and/or CY1 gastric cancer and found that conversion surgery was performed in 64
patients, among whom R0 resection was performed in 44 (69%).
Table 2 shows the promising results of several phase II clinical trials of i.p. taxanes after 2010. In these series,
the 1-year OS rates were 69% to 78%, with an MST of 16.2 to 24.6 months [21,24-27] . Notably, the possibility of
negative peritoneal cytology was very high at 81.8% to 97.0%.