Page 48 - Read Online
P. 48
Uchihara et al. J Cancer Metastasis Treat 2018;4:9 I http://dx.doi.org/10.20517/2394-4722.2017.81 Page 5 of 8
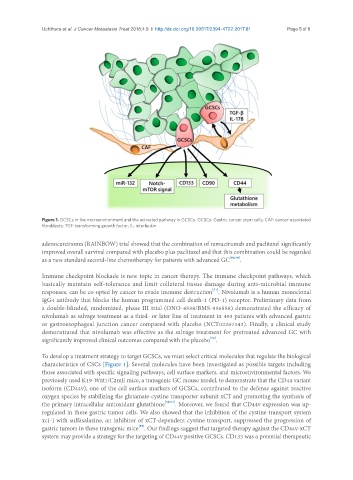
Figure 1. GCSCs in the microenvironment and the activated pathway in GCSCs. GCSCs: Gastric cancer stem cells; CAF: cancer-associated
fibroblasts; TGF: transforming growth factor; IL: interleukin
adenocarcinoma (RAINBOW) trial showed that the combination of ramucirumab and paclitaxel significantly
improved overall survival compared with placebo plus paclitaxel and that this combination could be regarded
as a new standard second-line chemotherapy for patients with advanced GC [49,50] .
Immune checkpoint blockade is new topic in cancer therapy. The immune checkpoint pathways, which
basically maintain self-tolerance and limit collateral tissue damage during anti-microbial immune
[51]
responses, can be co-opted by cancer to evade immune destruction . Nivolumab is a human monoclonal
IgG4 antibody that blocks the human programmed cell death-1 (PD-1) receptor. Preliminary data from
a double-blinded, randomized, phase III trial (ONO-4538/BMS-936558) demonstrated the efficacy of
nivolumab as salvage treatment as a third- or later line of treatment in 493 patients with advanced gastric
or gastroesophageal junction cancer compared with placebo (NCT02267343). Finally, a clinical study
demonstrated that nivolumab was effective as the salvage treatment for pretreated advanced GC with
[52]
significantly improved clinical outcomes compared with the placebo .
To develop a treatment strategy to target GCSCs, we must select critical molecules that regulate the biological
characteristics of CSCs [Figure 1]. Several molecules have been investigated as possible targets including
those associated with specific signaling pathways, cell surface markers, and microenvironmental factors. We
previously used K19-Wnt1/C2mE mice, a transgenic GC mouse model, to demonstrate that the CD44 variant
isoform (CD44v), one of the cell surface markers of GCSCs, contributed to the defense against reactive
oxygen species by stabilizing the glutamate-cystine transporter subunit xCT and promoting the synthesis of
the primary intracellular antioxidant glutathione [53,54] . Moreover, we found that CD44v expression was up-
regulated in these gastric tumor cells. We also showed that the inhibition of the cystine transport system
xc(-) with sulfasalazine, an inhibitor of xCT-dependent cystine transport, suppressed the progression of
[55]
gastric tumors in these transgenic mice . Our findings suggest that targeted therapy against the CD44v-xCT
system may provide a strategy for the targeting of CD44v positive GCSCs. CD133 was a potential therapeutic