Page 47 - Read Online
P. 47
Page 4 of 8 Uchihara et al. J Cancer Metastasis Treat 2018;4:9 I http://dx.doi.org/10.20517/2394-4722.2017.81
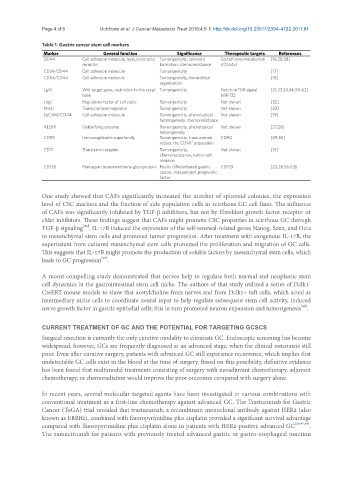
Table 1. Gastric cancer stem cell markers
Marker General function Significance Therapeutic targets References
CD44 Cell adhesion molecule, hyaluronic acid Tumorigenicity, spheroid Glutathione metabolism [16,28,58]
receptor formation, chemoresistance (CD44v)
CD24/CD44 Cell adhesion molecule Tumorigenicity [17]
CD54/CD44 Cell adhesion molecule Tumorigenicity, hierarchical [18]
organization
Lgr5 Wnt target gene, restriction to the crypt Tumorigenicity Notch-mTOR signal [21,23,24,46,59-62]
base miR-132
Lrig1 Regulatory factor of cell cycle Tumorigenicity Not shown [32]
Mist1 Transcriptional regulator Tumorigenicity Not shown [33]
EpCAM/CD44 Cell adhesion molecule Tumorigenicity, phenotypical Not shown [19]
heterogeneity, chemoresistance
ALDH1 Detoxifying enzyme Tumorigenicity, phenotypical Not shown [27,28]
heterogeneity
CD90 Immunoglobulin superfamily Tumorigenicity, trastuzumab CD90 [29,30]
+
reduce the CD90 population
CD71 Transferrin receptor Tumorigenicity, Not shown [31]
chemoresistance, tumor cell
invasion
CD133 Pentaspan transmembrane glycoprotein Poorly differentiated gastric CD133 [25,26,56,63]
cancer, independent prognostic
factor
One study showed that CAFs significantly increased the number of spheroid colonies, the expression
level of CSC markers and the fraction of side population cells in scirrhous GC cell lines. The influence
of CAFs was significantly inhibited by TGF-b inhibitors, but not by fibroblast growth factor receptor or
cMet inhibitors. These findings suggest that CAFs might promote CSC properties in scirrhous GC through
[44]
TGF-b signaling . IL-17B induced the expression of the self-renewal-related genes Nanog, Sox2, and Oct4
in mesenchymal stem cells and promoted tumor progression. After treatment with exogenous IL-17B, the
supernatant from cultured mesenchymal stem cells promoted the proliferation and migration of GC cells.
This suggests that IL-17B might promote the production of soluble factors by mesenchymal stem cells, which
leads to GC progression .
[45]
A recent compelling study demonstrated that nerves help to regulate both normal and neoplastic stem
cell dynamics in the gastrointestinal stem cell niche. The authors of that study utilized a series of Dclk1-
CreERT mouse models to show that acetylcholine from nerves and from Dclk1+ tuft cells, which acted as
intermediary niche cells to coordinate neural input to help regulate subsequent stem cell activity, induced
[46]
nerve growth factor in gastric epithelial cells; this in turn promoted neuron expansion and tumorigenesis .
CURRENT TREATMENT OF GC AND THE POTENTIAL FOR TARGETING GCSCS
Surgical resection is currently the only curative modality to eliminate GC. Endoscopic screening has become
widespread, however, GCs are frequently diagnosed at an advanced stage, when the clinical outcomeis still
poor. Even after curative surgery, patients with advanced GC still experience recurrence, which implies that
undetectable GC cells exist in the blood at the time of surgery. Based on this possibility, definitive evidence
has been found that multimodal treatments consisting of surgery with neoadjuvant chemotherapy, adjuvant
chemotherapy, or chemoradiation would improve the poor outcomes compared with surgery alone.
In recent years, several molecular-targeted agents have been investigated in various combinations with
conventional treatment as a first-line chemotherapy against advanced GC. The Trastuzumab for Gastric
Cancer (ToGA) trial revealed that trastuzumab, a recombinant monoclonal antibody against HER2 (also
known as ERBB2), combined with fluoropyrimidine plus cisplatin provided a significant survival advantage
compared with fluoropyrimidine plus cisplatin alone in patients with HER2-positive advanced GC [29,47,48] .
The ramucirumab for patients with previously treated advanced gastric or gastro-esophageal junction