Page 121 - Read Online
P. 121
Page 8 of 15 Kiyozumi et al. J Cancer Metastasis Treat 2018;4:31 I http://dx.doi.org/10.20517/2394-4722.2017.77
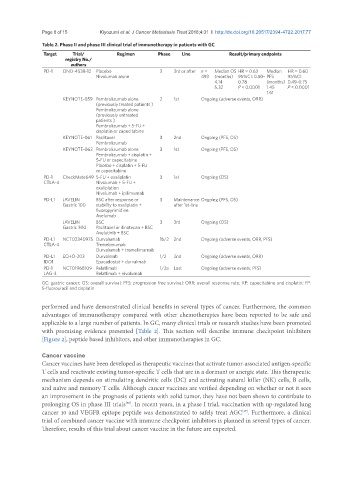
Table 2. Phase II and phase III clinical trial of immunotherapy in patients with GC
Target Trial/ Regimen Phase Line Result/primary endpoints
registry No./
authors
PD-1 ONO-4538-12 Placebo 3 3rd or after n = Median OS HR = 0.63 Median HR = 0.60
Nivolumab alone 493 (months) 95%CI: 0.50- PFS 95%CI:
4.14 0.78 (months) 0.49-0.75
5.32 P < 0.0001 1.45 P < 0.0001
1.61
KEYNOTE-059 Pembrolizumab alone 2 1st Ongoing (adverse events, ORR)
(previously treated patients )
Pembrolizumab alone
(previously untreated
patients )
Pembrolizumab + 5-FU +
cisplatin or capecitabine
KEYNOTE-061 Paclitaxel 3 2nd Ongoing (PFS, OS)
Pembrolizumab
KEYNOTE-062 Pembrolizumab alone 3 1st Ongoing (PFS, OS)
Pembrolizumab + cisplatin +
5-FU or capecitabine
Placebo + cisplatin + 5-FU
or capecitabine
PD-1 CheckMate649 5-FU + oxaliplatin 3 1st Ongoing (OS)
CTLA-4 Nivolumab + 5-FU +
oxaliplation
Nivolumab + ipilimumab
PD-L1 JAVELIN BSC after response or 3 Maintenance Ongoing (PFS, OS)
Gastric 100 stability to oxaliplatin + after 1st-line
fluoropyrimidine
Avelumab
JAVELIN BSC 3 3rd Ongoing (OS)
Gastric 300 Paclitaxel or ilinotecan + BSC
Avelutinib + BSC
PD-L1 NCT02340975 Durvalumab 1b/2 2nd Ongoing (adverse events, ORR, PFS)
CTLA-4 Tremelimumab
Durvalumab + tremelimumab
PD-L1 ECHO-203 Durvalmab 1/2 2nd Ongoing (adverse events, ORR)
IDO1 Epacadostat + durvalmab
PD-1 NCT01968109 Relatlimab 1/2a Last Ongoing (adverse events, PFS)
LAG-3 Relatlimab + nivolumab
GC: gastric cancer; OS: overall survival; PFS: progression free survival; ORR: overall response rate; XP: capecitabine and cisplatin; FP:
5-fluorouracil and cisplatin
performed and have demonstrated clinical benefits in several types of cancer. Furthermore, the common
advantages of immunotherapy compared with other chemotherapies have been reported to be safe and
applicable to a large number of patients. In GC, many clinical trials or research studies have been promoted
with promising evidence presented [Table 2]. This section will describe immune checkpoint inhibiters
[Figure 2], peptide based inhibitors, and other immunotherapies in GC.
Cancer vaccine
Cancer vaccines have been developed as therapeutic vaccines that activate tumor-associated antigen-specific
T cells and reactivate existing tumor-specific T cells that are in a dormant or anergic state. This therapeutic
mechanism depends on stimulating dendritic cells (DC) and activating natural killer (NK) cells, B cells,
and naïve and memory T cells. Although cancer vaccines are verified depending on whether or not it sees
an improvement in the prognosis of patients with solid tumor, they have not been shown to contribute to
prolonging OS in phase III trials . In recent years, in a phase I trial, vaccination with up-regulated lung
[36]
cancer 10 and VEGFR epitope peptide was demonstrated to safely treat AGC . Furthermore, a clinical
[37]
trial of combined cancer vaccine with immune checkpoint inhibitors is planned in several types of cancer.
Therefore, results of this trial about cancer vaccine in the future are expected.