Page 117 - Read Online
P. 117
Page 4 of 15 Kiyozumi et al. J Cancer Metastasis Treat 2018;4:31 I http://dx.doi.org/10.20517/2394-4722.2017.77
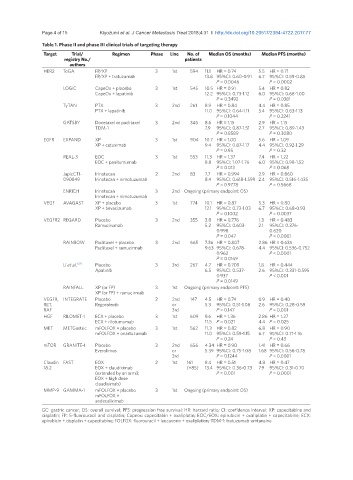
Table 1. Phase II and phase III clinical trials of targeting therapy
Target Trial/ Regimen Phase Line No. of Median OS (months) Median PFS (months)
registry No./ patients
authors
HER2 ToGA FP/XP 3 1st 594 11.1 HR = 0.74 5.5 HR = 0.71
FP/XP + tratuzumab 13.8 95%CI: 0.60-0.91 6.7 95%CI: 0.59-0.85
P = 0.0046 P = 0.0002
LOGiC CapeOx + placebo 3 1st 545 10.5 HR = 0.91 5.4 HR = 0.82
CapeOx + lapatinib 12.2 95%CI: 0.73-1.12 6.0 95%CI: 0.68-1.00
P = 0.3492 P = 0.0381
TyTAN PTX 3 2nd 261 8.9 HR = 0.84 4.4 HR = 0.85
PTX + lapatinib 11.0 95%CI: 0.64-1.11 5.4 95%CI: 0.63-1.13
P = 0.1044 P = 0.2241
GATSBY Docetaxel or paclitaxel 3 2nd 345 8.6 HR = 1.15 2.9 HR = 1.13
TDM-1 7.9 95%CI: 0.87-1.51 2.7 95%CI: 0.89-1.43
P = 0.8589 P = 0.3080
EGFR EXPAND XP 3 1st 904 10.7 HR = 1.00 5.6 HR = 1.09
XP + cetuximab 9.4 95%CI: 0.87-1.17 4.4 95%CI: 0.92-1.29
P = 0.95 P = 0.32
REAL-3 EOC 3 1st 553 11.3 HR = 1.37 7.4 HR = 1.22
EOC + panitumumab 8.8 95%CI: 1.07-1.76 6.0 95%CI: 0.98-1.52
P = 0.013 P = 0.068
JapicCTI- Irinotecan 2 2nd 83 7.7 HR = 0.994 2.9 HR = 0.860
090849 Irinotecan + nimotuzumab 8.4 95%CI: 0.618-1.599 2.4 95%CI: 0.516-1.435
P = 0.9778 P = 0.5668
ENRICH Irinotecan 3 2nd Ongoing (primary endpoint: OS)
Irinotecan + nimotuzumab
VEGF AVAGAST XP + placebo 3 1st 774 10.1 HR = 0.87 5.3 HR = 0.80
XP + bevacizumab 12.1 95%CI: 0.73-1.03 6.7 95%CI: 0.68-0.93
P = 0.1002 P = 0.0037
VEGFR2 REGARD Placebo 3 2nd 355 3.8 HR = 0.776 1.3 HR = 0.483
Ramucirumab 5.2 95%CI: 0.603- 2.1 95%CI: 0.376-
0.998 0.620
P = 0.047 P < 0.0001
RAINBOW Paclitaxel + placebo 3 2nd 665 7.36 HR = 0.807 2.86 HR = 0.635
Paclitaxel + ramucirmab 9.63 95%CI: 0.678- 4.4 95%CI: 0.536-0.752
0.962 P < 0.0001
P = 0.0169
Li et al. [25] Placebo 3 3rd 267 4.7 HR = 0.709 1.8 HR = 0.444
Apatinib 6.5 95%CI: 0.537- 2.6 95%CI: 0.331-0.595
0.937 P < 0.001
P = 0.0149
RAINFALL XP (or FP) 3 1st Ongoing (primary endpoint: PFS)
XP (or FP) + ramucirmab
VEGFR, INTEGRATE Placebo 2 2nd 147 4.5 HR = 0.74 0.9 HR = 0.40
RET, Regorafenib or 5.3 95%CI: 0.51-1.08 2.6 95%CI: 0.28-0.59
RAF 3rd P = 0.147 P < 0.001
HGF RILOMET-1 ECX + placebo 3 1st 609 9.6 HR = 1.36 2.86 HR = 1.27
ECX + rilotumumab 11.5 P = 0.021 4.4 P = 0.025
MET METGastric mFOLFOX + placebo 3 1st 562 11.3 HR = 0.82 6.8 HR = 0.90
mFOLFOX + onartuzumab 11.0 95%CI: 0.59-1.15 6.7 95%CI: 0.71-1.16
P = 0.24 P = 0.43
mTOR GRANITE-1 Placebo 3 2nd 656 4.34 HR = 0.90 1.41 HR = 0.66
Everolimus or 5.39 95%CI: 0.75-1.08 1.68 95%CI: 0.56-0.78
3rd P = 0.1244 P < 0.0001
Claudin FAST EOX 2 1st 161 8.4 HR = 0.51 4.8 HR = 0.47
18.2 EOX + claudiximab (+85) 13.4 95%CI: 0.36-0.73 7.9 95%CI: 0.31-0.70
(extended by an arm3; P < 0.001 P = 0.0001
EOX + high dose
claudiximab)
MMP-9 GAMMA-1 mFOLFOX + placebo 3 1st Ongoing (primary endpoint: OS)
mFOLFOX +
andecaliximab
GC: gastric cancer; OS: overall survival; PFS: progression free survival; HR: harzard ratio; CI: confidence interval; XP: capecitabine and
cisplatin; FP: 5-fluorouracil and cisplatin; Capeox: capecitabin + oxaliplatin; EOC/EOX: epirubicin + oxaliplatin + capecitabine; ECX:
epirubicin + cisplatin + capecitabine; FOLFOX: fluorouracil + leucovorin + oxaliplation; TDM-1: tratuzumab-emtansine