Page 55 - Read Online
P. 55
Page 6 of 13 Méndez-Sánchez et al. Hepatoma Res 2020;6:5 I http://dx.doi.org/10.20517/2394-5079.2019.29
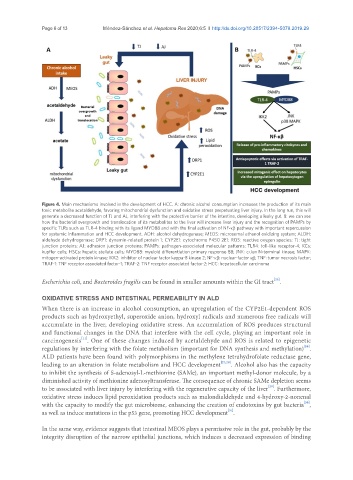
Figure 4. Main mechanisms involved in the development of HCC. A: chronic alcohol consumption increases the production of its main
toxic metabolite acetaldehyde, favoring mitochondrial dysfunction and oxidative stress perpetuating liver injury. In the long run, this will
generate a decreased function of TJ and AJ, interfering with the protective barrier of the intestine, developing a leaky gut. B: we can see
how the bacterial overgrowth and translocation of its metabolites to the liver will increase liver injury and the recognition of PAMPs by
specific TLRs such as TLR-4 binding with its ligand MYD88 and with the final activation of NF-κβ pathway with important repercussion
for systemic inflammation and HCC development. ADH: alcohol dehydrogenase; MEOS: microsomal ethanol oxidizing system; ALDH:
aldehyde dehydrogenase; DRP1: dynamin-related protein 1; CYP2E1: cytochrome P450 2E1; ROS: reactive oxygen species; TJ: tight
junction proteins; AJ: adhesion junction proteins; PAMPs: pathogen-associated molecular patterns; TLR4: toll-like receptor-4; KCs:
kupffer cells; HSCs: hepatic stellate cells; MYD88: myeloid differentiation primary response 88; JNK: c-Jun N-terminal kinase; MAPK:
mitogen-activated protein kinase; IKK2: inhibitor of nuclear factor kappa-B kinase 2; NF-κβ: nuclear-factor κβ; TNF: tumor necrosis factor;
TRAF-1: TNF receptor associated factor-1; TRAF-2: TNF receptor associated factor-2; HCC: hepatocellular carcinoma
[25]
Escherichia coli, and Bacteroides fragilis can be found in smaller amounts within the GI tract .
OXIDATIVE STRESS AND INTESTINAL PERMEABILITY IN ALD
When there is an increase in alcohol consumption, an upregulation of the CYP2E1-dependent ROS
products such as hydroxyethyl, superoxide anion, hydroxyl radicals and numerous free radicals will
accumulate in the liver, developing oxidative stress. An accumulation of ROS produces structural
and functional changes in the DNA that interfere with the cell cycle, playing an important role in
[11]
carcinogenesis . One of these changes induced by acetaldehyde and ROS is related to epigenetic
[26]
regulations by interfering with the folate metabolism (important for DNA synthesis and methylation) .
ALD patients have been found with polymorphisms in the methylene tetrahydrofolate reductase gene,
leading to an alteration in folate metabolism and HCC development [27,28] . Alcohol also has the capacity
to inhibit the synthesis of S-adenosyl-L-methionine (SAMe), an important methyl-donor molecule, by a
diminished activity of methionine adenosyltransferase. The consequence of chronic SAMe depletion seems
[29]
to be associated with liver injury by interfering with the regenerative capacity of the liver . Furthermore,
oxidative stress induces lipid peroxidation products such as malondialdehyde and 4-hydroxy-2-nonenal
[30]
with the capacity to modify the gut microbiome, enhancing the creation of endotoxins by gut bacteria ,
[31]
as well as induce mutations in the p53 gene, promoting HCC development .
In the same way, evidence suggests that intestinal MEOS plays a permissive role in the gut, probably by the
integrity disruption of the narrow epithelial junctions, which induces a decreased expression of binding