Page 27 - Read Online
P. 27
Bourinbaiar et al. Hepatoma Res 2020;6:2 I http://dx.doi.org/10.20517/2394-5079.2019.25 Page 5 of 8
-6
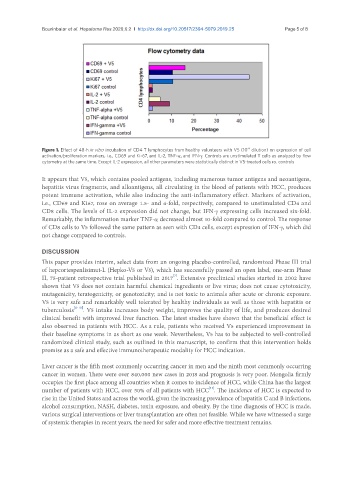
Figure 1. Effect of 48-h in vitro incubation of CD4 T lymphocytes from healthy volunteers with V5 (10 dilution) on expression of cell
activation/proliferation markers, i.e., CD69 and Ki-67, and IL-2, TNF-α, and IFN-γ. Controls are unstimulated T cells as analyzed by flow
cytometry at the same time. Except IL-2 expression, all other parameters were statistically distinct in V5-treated cells vs. controls
It appears that V5, which contains pooled antigens, including numerous tumor antigens and neoantigens,
hepatitis virus fragments, and alloantigens, all circulating in the blood of patients with HCC, produces
potent immune activation, while also inducing the anti-inflammatory effect. Markers of activation,
i.e., CD69 and Ki67, rose on average 1.5- and 4-fold, respectively, compared to unstimulated CD4 and
CD8 cells. The levels of IL-2 expression did not change, but IFN-γ expressing cells increased six-fold.
Remarkably, the inflammation marker TNF-α decreased almost 10-fold compared to control. The response
of CD8 cells to V5 followed the same pattern as seen with CD4 cells, except expression of IFN-γ, which did
not change compared to controls.
DISCUSSION
This paper provides interim, select data from an ongoing placebo-controlled, randomized Phase III trial
of hepcortespenlisimut-L (Hepko-V5 or V5), which has successfully passed an open label, one-arm Phase
[7]
II, 75-patient retrospective trial published in 2017 . Extensive preclinical studies started in 2002 have
shown that V5 does not contain harmful chemical ingredients or live virus; does not cause cytotoxicity,
mutagenicity, teratogenicity, or genotoxicity; and is not toxic to animals after acute or chronic exposure.
V5 is very safe and remarkably well tolerated by healthy individuals as well as those with hepatitis or
tuberculosis [8-10] . V5 intake increases body weight, improves the quality of life, and produces desired
clinical benefit with improved liver function. The latest studies have shown that the beneficial effect is
also observed in patients with HCC. As a rule, patients who received V5 experienced improvement in
their baseline symptoms in as short as one week. Nevertheless, V5 has to be subjected to well-controlled
randomized clinical study, such as outlined in this manuscript, to confirm that this intervention holds
promise as a safe and effective immunotherapeutic modality for HCC indication.
Liver cancer is the fifth most commonly occurring cancer in men and the ninth most commonly occurring
cancer in women. There were over 840,000 new cases in 2018 and prognosis is very poor. Mongolia firmly
occupies the first place among all countries when it comes to incidence of HCC, while China has the largest
[11]
number of patients with HCC, over 50% of all patients with HCC . The incidence of HCC is expected to
rise in the United States and across the world, given the increasing prevalence of hepatitis C and B infections,
alcohol consumption, NASH, diabetes, toxin exposure, and obesity. By the time diagnosis of HCC is made,
various surgical interventions or liver transplantation are often not feasible. While we have witnessed a surge
of systemic therapies in recent years, the need for safer and more effective treatment remains.