Page 26 - Read Online
P. 26
Page 4 of 8 Bourinbaiar et al. Hepatoma Res 2020;6:2 I http://dx.doi.org/10.20517/2394-5079.2019.25
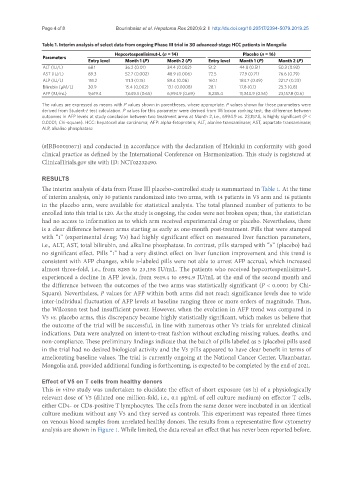
Table 1. Interim analysis of select data from ongoing Phase III trial in 30 advanced-stage HCC patients in Mongolia
Hepcortespenlisimut-L (n = 14) Placebo (n = 16)
Parameters
Entry level Month 1 (P) Month 2 (P) Entry level Month 1 (P) Month 2 (P)
ALT (IU/L) 68.1 36.2 (0.01) 34.4 (0.002) 51.2 44.8 (0.51) 50.3 (0.93)
AST (IU/L) 89.3 52.7 (0.002) 48.9 (0.006) 72.5 77.9 (0.71) 76.6 (0.79)
ALP (IU/L) 151.2 111.3 (0.15) 89.4 (0.06) 160.1 184.7 (0.49) 221.7 (0.23)
Bilirubin (µM/L) 30.9 15.4 (0.002) 13.1 (0.0008) 28.1 17.8 (0.1) 25.3 (0.8)
AFP (IU/mL) 9,619.4 7,649.3 (0.65) 6,994.9 (0.69) 8,285.4 11,340.9 (0.55) 23,157.8 (0.6)
The values are expressed as means with P values shown in parentheses, where appropriate. P values shown for these parameters were
derived from Student-t test calculation. P values for this parameter were derived from Wilcoxon ranking test; the difference between
outcomes in AFP levels at study conclusion between two treatment arms at Month 2, i.e., 6994.9 vs. 23,157.8, is highly significant (P <
0.0001; Chi-square). HCC: hepatocellular carcinoma; AFP: alpha-fetoprotein; ALT, alanine transaminase; AST, aspartate transaminase;
ALP, alkaline phosphatase
(#IRB00010671) and conducted in accordance with the declaration of Helsinki in conformity with good
clinical practice as defined by the International Conference on Harmonization. This study is registered at
ClinicalTrials.gov site with ID: NCT02232490.
RESULTS
The interim analysis of data from Phase III placebo-controlled study is summarized in Table 1. At the time
of interim analysis, only 30 patients randomized into two arms, with 14 patients in V5 arm and 16 patients
in the placebo arm, were available for statistical analysis. The total planned number of patients to be
enrolled into this trial is 120. As the study is ongoing, the codes were not broken open; thus, the statistician
had no access to information as to which arm received experimental drug or placebo. Nevertheless, there
is a clear difference between arms starting as early as one-month post-treatment. Pills that were stamped
with “1” (experimental drug; V5) had highly significant effect on measured liver function parameters,
i.e., ALT, AST, total bilirubin, and alkaline phosphatase. In contrast, pills stamped with “5” (placebo) had
no significant effect. Pills “1” had a very distinct effect on liver function improvement and this trend is
consistent with AFP changes, while 5-labeled pills were not able to arrest AFP accrual, which increased
almost three-fold, i.e., from 8285 to 23,158 IU/mL. The patients who received hepcortespenlisimut-L
experienced a decline in AFP levels, from 9619.4 to 6994.9 IU/mL at the end of the second month and
the difference between the outcomes of the two arms was statistically significant (P < 0.0001 by Chi-
Square). Nevertheless, P values for AFP within both arms did not reach significance levels due to wide
inter-individual fluctuation of AFP levels at baseline ranging three or more orders of magnitude. Thus,
the Wilcoxon test had insufficient power. However, when the evolution in AFP trend was compared in
V5 vs. placebo arms, this discrepancy became highly statistically significant, which makes us believe that
the outcome of the trial will be successful, in line with numerous other V5 trials for unrelated clinical
indications. Data were analyzed on intent-to-treat fashion without excluding missing values, deaths, and
non-compliance. These preliminary findings indicate that the batch of pills labeled as 5 (placebo) pills used
in the trial had no desired biological activity and the V5 pills appeared to have clear benefit in terms of
ameliorating baseline values. The trial is currently ongoing at the National Cancer Center, Ulaanbaatar,
Mongolia and, provided additional funding is forthcoming, is expected to be completed by the end of 2021.
Effect of V5 on T cells from healthy donors
This in vitro study was undertaken to elucidate the effect of short exposure (48 h) of a physiologically
relevant dose of V5 (diluted one million-fold, i.e., 0.1 µg/mL of cell culture medium) on effector T cells,
either CD4- or CD8-positive T lymphocytes. The cells from the same donor were incubated in an identical
culture medium without any V5 and they served as controls. This experiment was repeated three times
on venous blood samples from unrelated healthy donors. The results from a representative flow cytometry
analysis are shown in Figure 1. While limited, the data reveal an effect that has never been reported before.