Page 294 - Read Online
P. 294
Page 6 of 13 Harrod et al. Hepatoma Res 2019;5:28 I http://dx.doi.org/10.20517/2394-5079.2019.15
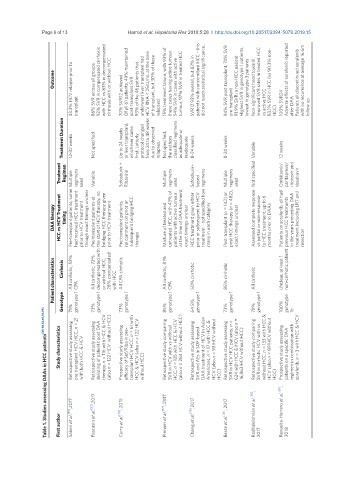
33.3% HCV relapse prior to transplant 86% SVR across all groups cirrhosis with or without HCC 70% SVR12 achieved post-transplant SVR 93% of the 46 patients that underwent liver transplant had from 24 to 48 weeks HCV RNA < 25IU/mL at the time of transplant, but 30% of these relapsed. 54% SVR in cases with active SVR12 93% overall, but 82% in pre-transplant 91.9% SVR in non-HCC patient lowest in genotype 3 patients Non-significant
Outcome 92% SVR in compensated cirrhosis with HCC vs 83% in decompensated Of all 61 patients, 49% maintained 15% treatment failure, with 93% of these cases having active tumour tumour, 97% SVR in treated HCC patients with concomitant HCC - this did not reach statistical significance. 94% SVR post-transplant, 74% SVR Highest SVR in genotype 1 patients, improved SVR rates in treated HCC 83.1% SVR in HCC (vs 90.3% non- Adverse effe
Treatment Duration 12-32 weeks Not specified Up to 24 weeks or liver transplant, whichever came first. Latterly protocol changed due to observed relapses. Not specified; the authors classified regimens as adequate or inadequate 8-24 weeks 8-24 weeks Variable 12 weeks
Treatment Regimen regimens used Variable Sofosbuvir + Ribavirin Multiple regimens used Sofosbuvir- based Multiple regimens used Not specified Ombitasvir/ paritaprevir/ ritonavir and dasabuvir
DAA therapy HCC vs HCV Treatment timing Pre-transplant patients, some Multiple had received HCC treatment prior to HCV treatment though exact timings unclear Pre-transplant patients at decompensated with the start of HCV therapy, no bridging HCC therapy given prior to HCV treatment Pre-transplant patients, no comment on prior or subsequent bridging HCC therapy Mixture of treated and untreated HCC, with 43% of patients with active tum
Patient characteristics Cirrhosis Genotype All cirrhotic, 52% 71% CPA genotype 1 All cirrhotic, 72% 73% genotype 1 or without HCC, 28% compensated with HCC All CPA cirrhotic 73% genotype 1 All cirrhotic, 81% 85% CPA genotype 1 50% cirrhotic 64.5% genotype 1 85% cirrhotic 73% genotype 1 All cirrhotic 78% genotype 1 Unclear, at least 1 100% non-cirrhotic patient genotype 1b
Table 1. Studies assessing DAAs in HCC patients [35-38,52,69,78,79]
Study characteristics Retrospective study assessing pre transplant HCV HCC, n = 21 with both HCC & HCV Retrospective study assessing delisting of patients post DAA therapy, n = 116 with HCC & HCV (also n = 122 HCV without HCC) Prospective study assessing efficacy of Sof/Riba in pre- transplant HCV HCC, n = 61 with HCC & HCV (also n = 122 HCV without HCC) Retrospective study comparing SVR in HCV with vs without HCC, n = 135 with HCC &
First author Saberi et al. [35] ,2017 Pascasio et al. [52] ,2017 Curry et al. [78] , 2015 Prenner et al. [37] , 2017 Chang et al. [79] ,2017 Beste et al. [36] , 2017 Radhakrishnan et al. [38] , 2017 Revuelta-Herrero et al. [69] , 2018