Page 221 - Read Online
P. 221
Page 6 of 9 Tang et al. Hepatoma Res 2019;5:19 I http://dx.doi.org/10.20517/2394-5079.2019.07
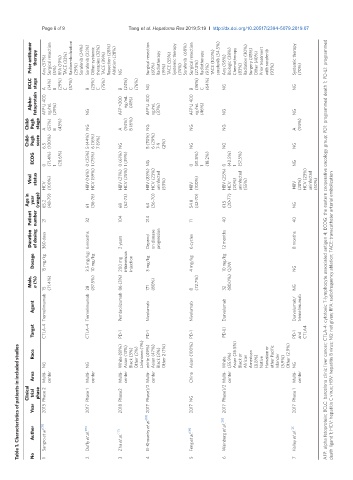
Prior antitumor therapy Any (57%) Surgical resection (5%) RFA (19%) TACE (33%) Radioembolization (29%) Sorafenib (24%) Sorafenib (22%) Other systemic therapies (32%) TACE (39%) Resection (28%) Ablation (28%) Surgical resection (60%) Radiotherapy (19%) TACE (55%) Systemic therapy (74%) Sorafenib (68%) Surgical resection (27.3%) Radiotherapy (9.1%) TACE (100%) sorafenib (54.5%) Any (95%) Biologic (38%) Chemotherapy (83
BCLC stage A (14%) B (29%) C (57%) B (25%) C (75%) NG B (24%) C (76%) NG B (36%) C (64%) NG NG
Alpha- fetoprotein AFP ≥ 400 ng/mL (29%) NG AFP >200 ng/mL (41%) AFP ≥ 400 ng/mL (37%) AFP ≥ 400 ng/mL (46%) NG NG
Child- Pugh stage A (57%) B (43%) NG A (94%) B (6%) NG NG NG A (93%)
Child- Pugh score 6.5 (100%) 5 (44%) 6 (16%) 7 (9%) NG 5 (70%) 6 (29%) 7-9 (2%) NG NG NG
ECOG 0 (71.4%) 1 (28.6%) 0 (25%) 1 (75%) 0 (61%) 1 (39%) NG 0 (81.8%) 1 (18.2%) 0 (42.5%) 1 (57.5%) NG
Viral status HBV (16%) HCV (59%) HBV (21%) HCV (25%) HBV (83%) HCV (23%) uninfected HBV (25%) uninfected HCV (23%) uninfected
HCV (100%) (53%) HBV (100%) HCV (20%) (55%) HBV (28%) (50%)
Age in year (range) 65.2 (48-79) 61 (36-76) 68 (62-73) 64 (56-70) 54.8 (42-70) 61.5 (20-77) NG
Patient number 21 32 104 214 11 40 40
Duration of dosing 360 days 6 months 2 years Depend on disease progression 6 cycles 12 months 8 months AFP: alpha fetoprotein; BCLC: barcelona clinic liver cancer; CTLA-4: cytotoxic T-lymphocyte associated antigen 4; ECOG: the eastern cooperative oncology group; PD1: programmed death 1; PD-L1: programmed
Dosage 15 mg/kg 3.5 mg/kg; 10 mg/kg 200 mg intravenous injection 3 mg/kg 4 mg/kg 10 mg/kg Q2W NG
Male, n (%) 15 (71.4%) 28 (87.5%) 86 (3%) 171 (80%) 8 (72.7%) 32 (80.0%) NG
Tremelimumab Tremelimumab Nivolumab Nivolumab Durvalumab Durvalumab/ tremelimumab death ligand 1; HCV: hepatitis C virus; HBV: hepatitis B virus; NG: not given; RFA: radiofrequency ablation; TACE: transcatheter arterial embolization
Agent Pembrolizumab
Target CTLA-4 CTLA-4 PD-1 PD-1 PD-1 PD-L1 PD-1 and CTLA4
Table 1. Characteristics of patients in included studies
Race White (81%) Asian (13%) Black (3%) Other (2%) Unknown (1%) white (49%) Asian (47%) Black (3%) Other 2 (1%) Asian (100%) Asian (26.5%) American Hawaiian or other Pacific Other (2.9%)
NG NG White (55.9%) Black or African (8.8%) Native Islander (5.9%) NG
Area Multi- center Multi- center Multi- center Multi- center China center Multi- center
Clinical trial phase Phase 2 Phase 1 Phase2 Phase1/2 NG Phase1/2 Multi- Phase 1
Year 2013 2017 2018 2017 2017 2017 2017
Author Sangro et al. [15] Duffy et al. [16] Zhu et al. [17] El-Khoueiry et al. [18] Feng et al. [19] Wainberg et al. [20] Kelley et al. [21]
No 1 2 3 4 5 6 7