Page 785 - Read Online
P. 785
Zanetto et al. Hepatoma Res 2018;4:70 I http://dx.doi.org/10.20517/2394-5079.2018.102 Page 5 of 16
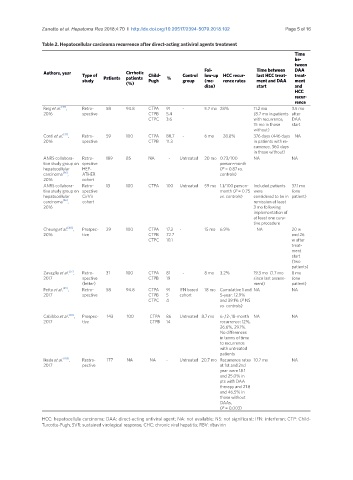
Table 2. Hepatocellular carcinoma recurrence after direct-acting antiviral agents treatment
Time
be-
tween
Authors, year Type of Cirrhotic Child- Control Fol- Time between DAA
low-up HCC recur-
last HCC treat-
treat-
study Patients patients Pugh % group (me- rence rates ment and DAA ment
(%)
dian) start and
HCC
recur-
rence
Reig et al. [35] , Retro- 58 94.8 CTPA 91 - 5.7 mo 28% 11.2 mo 3.5 mo
2016 spective CTPB 5.4 (8.7 mo in patients after
CTPC 3.6 with recurrence, DAA
15 mo in those start
without)
Conti et al. [37] , Retro- 59 100 CTPA 88.7 - 6 mo 28.8% 376 days (446 days NA
2016 spective CTPB 11.3 in patients with re-
currence, 360 days
in those without)
ANRS collabora- Retro- 189 85 NA - Untreated 20 mo 0.73/100 NA NA
tive study group on spective person-month
hepatocellular HEP- (P = 0.87 vs.
carcinoma [56] , ATHER controls)
2016 cohort
ANRS collabora- Retro- 13 100 CTPA 100 Untreated 59 mo 1.1/100 person- Included patients 37.1 mo
tive study group on spective month (P = 0.75 were (one
hepatocellular CirVir vs. controls) considered to be in patient)
carcinoma [56] , cohort remission at least
2016 3 mo following
implementation of
at least one cura-
tive procedure
Cheung et al. [40] , Prospec- 29 100 CTPA 17.2 - 15 mo 6.9% NA 20 w
2016 tive CTPB 72.7 and 26
CTPC 10.1 w after
treat-
ment
start
(two
patients)
Zavaglia et al. [57] , Retro- 31 100 CTPA 81 - 8 mo 3.2% 19.3 mo (1.7 mo 8 mo
2017 spective CTPB 19 since last assess- (one
(letter) ment) patient)
Petta et al. [59] , Retro- 58 94.8 CTPA 91 IFN based 18 mo Cumulative 1 and NA NA
2017 spective CTPB 5 cohort 5-year: 12.9%
CTPC 4 and 39.1% (P NS
vs. controls)
Cabibbo et al. [58] , Prospec- 143 100 CTPA 86 Untreated 8.7 mo 6-,12-,18-month NA NA
2017 tive CTPB 14 recurrence: 12%,
26.6%, 29.1%.
No differences
in terms of time
to recurrence
with untreated
patients
Ikeda et al. [105] , Restro- 177 NA NA - Untreated 20.7 mo Recurrence rates 10.7 mo NA
2017 pective at 1st and 2nd
year were 18.1
and 25.0% in
pts with DAA
therapy and 21.8
and 46.5% in
those without
DAAs,
(P = 0.003)
HCC: hepatocellula carcinoma; DAA: direct-acting antiviral agent; NA: not available; NS: not significant; IFN: interferon; CTP: Child-
Turcotte-Pugh; SVR: sustained virological response; CHC: chronic viral hepatitis; RBV: ribavirin