Page 59 - Read Online
P. 59
Page 8 of 17 Chan et al. Hepatoma Res 2018;4:5 I http://dx.doi.org/10.20517/2394-5079.2017.49
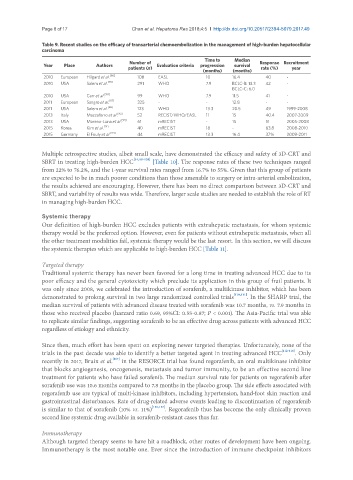
Table 9. Recent studies on the efficacy of transarterial chemoembolization in the management of high-burden hepatocellular
carcinoma
Time to Median
Number of Response Recruitment
Year Place Authors Evaluation criteria progression survival
patients (n) rate (%) year
(months) (months)
2010 European Hilgard et al. [86] 108 EASL 10 16.4 40 -
2010 USA Salem et al. [89] 291 WHO 7.9 BCLC-B: 13.3 42 -
BCLC-C: 6.0
2010 USA Carr et al. [90] 99 WHO 7.9 11.5 41 -
2011 European Sangro et al. [87] 325 - - 12.8 - -
2011 USA Salem et al. [88] 123 WHO 13.3 20.5 49 1999-2008
2013 Italy Mazzaferro et al. [92] 52 RECIST/WHO/EASL 11 15 40.4 2007-2009
2013 USA Moreno-Luna et al. [93] 61 mRECIST - 15 51 2005-2008
2015 Korea Kim et al. [91] 40 mRECIST 18 - 63.8 2008-2010
2015 Germany El Fouly et al. [94] 44 mRECIST 13.3 16.4 37% 2009-2011
Multiple retrospective studies, albeit small scale, have demonstrated the efficacy and safety of 3D-CRT and
SBRT in treating high-burden HCC [54,98-109] [Table 10]. The response rates of these two techniques ranged
from 22% to 76.2%, and the 1-year survival rates ranged from 16.7% to 55%. Given that this group of patients
are expected to be in much poorer conditions than those amenable to surgery or intra-arterial embolization,
the results achieved are encouraging. However, there has been no direct comparison between 3D-CRT and
SBRT, and variability of results was wide. Therefore, larger scale studies are needed to establish the role of RT
in managing high-burden HCC.
Systemic therapy
Our definition of high-burden HCC excludes patients with extrahepatic metastasis, for whom systemic
therapy would be the preferred option. However, even for patients without extrahepatic metastasis, when all
the other treatment modalities fail, systemic therapy would be the last resort. In this section, we will discuss
the systemic therapies which are applicable to high-burden HCC [Table 11].
Targeted therapy
Traditional systemic therapy has never been favored for a long time in treating advanced HCC due to its
poor efficacy and the general cytotoxicity which preclude its application in this group of frail patients. It
was only since 2008, we celebrated the introduction of sorafenib, a multikinase inhibitor, which has been
demonstrated to prolong survival in two large randomized controlled trials [110,111] . In the SHARP trial, the
median survival of patients with advanced disease treated with sorafenib was 10.7 months, vs. 7.9 months in
those who received placebo (harzard ratio 0.69, 95%CI: 0.55-0.87; P < 0.001). The Asia-Pacific trial was able
to replicate similar findings, suggesting sorafenib to be an effective drug across patients with advanced HCC
regardless of etiology and ethnicity.
Since then, much effort has been spent on exploring newer targeted therapies. Unfortunately, none of the
trials in the past decade was able to identify a better targeted agent in treating advanced HCC [112-116] . Only
[117]
recently in 2017, Bruix et al. in the RESORCE trial has found regorafenib, an oral multikinase inhibitor
that blocks angiogenesis, oncogenesis, metastasis and tumor immunity, to be an effective second line
treatment for patients who have failed sorafenib. The median survival rate for patients on regorafenib after
sorafenib use was 10.6 months compared to 7.8 months in the placebo group. The side effects associated with
regorafenib use are typical of multi-kinase inhibitors, including hypertension, hand-foot skin reaction and
gastrointestinal disturbances. Rate of drug-related adverse events leading to discontinuation of regorafenib
is similar to that of sorafenib (10% vs. 11%) [110,117] . Regorafenib thus has become the only clinically proven
second line systemic drug available in sorafenib-resistant cases thus far.
Immunotherapy
Although targeted therapy seems to have hit a roadblock, other routes of development have been ongoing.
Immunotherapy is the most notable one. Ever since the introduction of immune checkpoint inhibitors