Page 448 - Read Online
P. 448
Farghaly et al. Hepatoma Res 2018;4:41 I http://dx.doi.org/10.20517/2394-5079.2018.30 Page 5 of 10
A B
C D
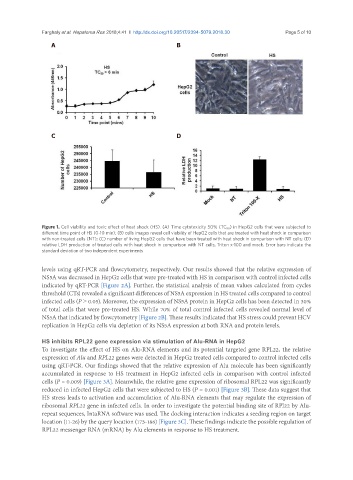
Figure 1. Cell viability and toxic effect of heat shock (HS). (A) Time cytotoxicity 50% (TC 50 ) in HepG2 cells that were subjected to
different time point of HS (0-10 min); (B) cells images reveal cell viability of HepG2 cells that are treated with heat shock in comparison
with non-treated cells (NT); (C) number of living HepG2 cells that have been treated with heat shock in comparison with NT cells; (D)
relative LDH production of treated cells with heat shock in comparison with NT cells, Triton x-100 and mock. Error bars indicate the
standard deviation of two independent experiments
levels using qRT-PCR and flowcytometry, respectively. Our results showed that the relative expression of
NS5A was decreased in HepG2 cells that were pre-treated with HS in comparison with control infected cells
indicated by qRT-PCR [Figure 2A]. Further, the statistical analysis of mean values calculated from cycles
threshold (CTs) revealed a significant differences of NS5A expression in HS treated cells compared to control
infected cells (P ˃ 0.05). Moreover, the expression of NS5A protein in HepG2 cells has been detected in 30%
of total cells that were pre-treated HS. While 70% of total control infected cells revealed normal level of
NS5A that indicated by flowcytometry [Figure 2B]. These results indicated that HS stress could prevent HCV
replication in HepG2 cells via depletion of its NS5A expression at both RNA and protein levels.
HS inhibits RPL22 gene expression via stimulation of Alu-RNA in HepG2
To investigate the effect of HS on Alu-RNA elements and its potential targeted gene RPL22, the relative
expression of Alu and RPL22 genes were detected in HepG2 treated cells compared to control infected cells
using qRT-PCR. Our findings showed that the relative expression of Alu molecule has been significantly
accumulated in response to HS treatment in HepG2 infected cells in comparison with control infected
cells (P = 0.009) [Figure 3A]. Meanwhile, the relative gene expression of ribosomal RPL22 was significantly
reduced in infected HepG2 cells that were subjected to HS (P = 0.001) [Figure 3B]. These data suggest that
HS stress leads to activation and accumulation of Alu-RNA elements that may regulate the expression of
ribosomal RPL22 gene in infected cells. In order to investigate the potential binding site of RPl22 by Alu-
repeat sequences, IntaRNA software was used. The docking interaction indicates a seeding region on target
location (11-26) by the query location (173-186) [Figure 3C]. These findings indicate the possible regulation of
RPL22 messenger RNA (mRNA) by Alu elements in response to HS treatment.