Page 67 - Read Online
P. 67
Page 6 of 10 Vezeridis et al. Hepatoma Res 2020;6:53 I http://dx.doi.org/10.20517/2394-5079.2020.36
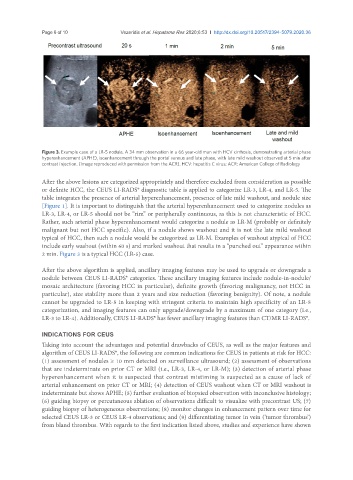
Figure 3. Example case of a LR-5 nodule. A 34 mm observation in a 66 year-old man with HCV cirrhosis, demonstrating arterial phase
hyperenhancement (APHE), isoenhancement through the portal venous and late phase, with late mild washout observed at 5 min after
contrast injection. (Image reproduced with permission from the ACR). HCV: hepatitis C virus; ACR: American College of Radiology
After the above lesions are categorized appropriately and therefore excluded from consideration as possible
or definite HCC, the CEUS LI-RADS® diagnostic table is applied to categorize LR-3, LR-4, and LR-5. The
table integrates the presence of arterial hyperenhancement, presence of late mild washout, and nodule size
[Figure 1]. It is important to distinguish that the arterial hyperenhancement used to categorize nodules as
LR-3, LR-4, or LR-5 should not be “rim” or peripherally continuous, as this is not characteristic of HCC.
Rather, such arterial phase hyperenhancement would categorize a nodule as LR-M (probably or definitely
malignant but not HCC specific). Also, if a nodule shows washout and it is not the late mild washout
typical of HCC, then such a nodule would be categorized as LR-M. Examples of washout atypical of HCC
include early washout (within 60 s) and marked washout that results in a “punched out” appearance within
2 min. Figure 3 is a typical HCC (LR-5) case.
After the above algorithm is applied, ancillary imaging features may be used to upgrade or downgrade a
nodule between CEUS LI-RADS® categories. These ancillary imaging features include nodule-in-nodule/
mosaic architecture (favoring HCC in particular), definite growth (favoring malignancy, not HCC in
particular), size stability more than 2 years and size reduction (favoring benignity). Of note, a nodule
cannot be upgraded to LR-5 in keeping with stringent criteria to maintain high specificity of an LR-5
categorization, and imaging features can only upgrade/downgrade by a maximum of one category (i.e.,
LR-3 to LR-4). Additionally, CEUS LI-RADS® has fewer ancillary imaging features than CT/MR LI-RADS®.
INDICATIONS FOR CEUS
Taking into account the advantages and potential drawbacks of CEUS, as well as the major features and
algorithm of CEUS LI-RADS®, the following are common indications for CEUS in patients at risk for HCC:
(1) assessment of nodules ≥ 10 mm detected on surveillance ultrasound; (2) assessment of observations
that are indeterminate on prior CT or MRI (i.e., LR-3, LR-4, or LR-M); (3) detection of arterial phase
hyperenhancement when it is suspected that contrast mistiming is suspected as a cause of lack of
arterial enhancement on prior CT or MRI; (4) detection of CEUS washout when CT or MRI washout is
indeterminate but shows APHE; (5) further evaluation of biopsied observation with inconclusive histology;
(6) guiding biopsy or percutaneous ablation of observations difficult to visualize with precontrast US; (7)
guiding biopsy of heterogeneous observations; (8) monitor changes in enhancement pattern over time for
selected CEUS LR-3 or CEUS LR-4 observations; and (9) differentiating tumor in vein (‘tumor thrombus’)
from bland thrombus. With regards to the first indication listed above, studies and experience have shown