Page 77 - Read Online
P. 77
Song et al. Hepatoma Res 2020;6:27 I http://dx.doi.org/10.20517/2394-5079.2020.05 Page 5 of 15
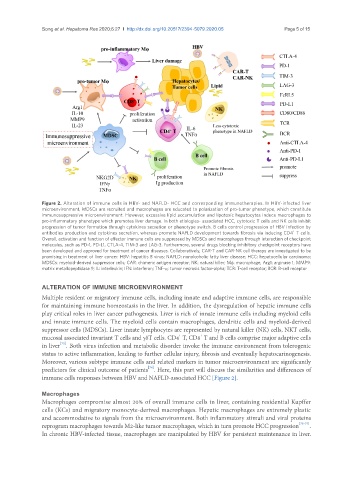
Figure 2. Alteration of immune cells in HBV- and NAFLD- HCC and corresponding immunotherapies. In HBV-infected liver
microenvironment, MDSCs are recruited and macrophages are educated to polarization of pro-tumor phenotype, which constitute
immunosuppressive microenvironment. However, excessive lipid accumulation and lipotoxic hepatocytes induce macrophages to
pro-inflammatory phenotype which promotes liver damage. In both etiologies- associated HCC, cytotoxic T cells and NK cells inhibit
progression of tumor formation through cytokines secretion or phenotype switch. B cells control progression of HBV infection by
+
antibodies production and cytokines secretion, whereas promote NAFLD development towards fibrosis via inducing CD4 T cells.
Overall, activation and function of effector immune cells are suppressed by MDSCs and macrophages through interaction of checkpoint
molecules, such as PD-1, PD-L1, CTLA-4, TIM-3 and LAG-3. Furthermore, several drugs blocking inhibitory checkpoint receptors have
been developed and approved for treatment of cancer diseases. Collaboratively, CAR-T and CAR-NK cell therapy are investigated to be
promising in treatment of liver cancer. HBV: hepatitis B virus; NAFLD: nonalcoholic fatty liver disease; HCC: hepatocellular carcinoma;
MDSCs: myeloid-derived suppressor cells; CAR: chimeric antigen receptor; NK: natural killer; Mφ: macrophage; Arg1: arginase 1; MMP9:
matrix metallopeptidase 9; IL: interleukin; IFN: interferon; TNF-α: tumor necrosis factor-alpha; TCR: T-cell receptor; BCR: B-cell receptor
ALTERATION OF IMMUNE MICROENVIRONMENT
Multiple resident or migratory immune cells, including innate and adaptive immune cells, are responsible
for maintaining immune homeostasis in the liver. In addition, the dysregulation of hepatic immune cells
play critical roles in liver cancer pathogenesis. Liver is rich of innate immune cells including myeloid cells
and innate immune cells. The myeloid cells contain macrophages, dendritic cells and myeloid-derived
suppressor cells (MDSCs). Liver innate lymphocytes are represented by natural killer (NK) cells, NKT cells,
+
+
mucosal associated invariant T cells and γδT cells. CD4 T, CD8 T and B cells comprise major adaptive cells
[73]
in liver . Both virus infection and metabolic disorder invoke the immune environment from tolerogenic
status to active inflammation, leading to further cellular injury, fibrosis and eventually hepatocarinogenesis.
Moreover, various subtype immune cells and related markers in tumor microenvironment are significantly
[74]
predictors for clinical outcome of patients . Here, this part will discuss the similarities and differences of
immune cells responses between HBV and NAFLD-associated HCC [Figure 2].
Macrophages
Macrophages compromise almost 20% of overall immune cells in liver, containing residential Kupffer
cells (KCs) and migratory monocyte-derived macrophages. Hepatic macrophages are extremely plastic
and accommodative to signals from the microenvironment. Both inflammatory stimuli and viral proteins
reprogram macrophages towards M2-like tumor macrophages, which in turn promote HCC progression [75-77] .
In chronic HBV-infected tissue, macrophages are manipulated by HBV for persistent maintenance in liver.