Page 75 - Read Online
P. 75
Song et al. Hepatoma Res 2020;6:27 I http://dx.doi.org/10.20517/2394-5079.2020.05 Page 3 of 15
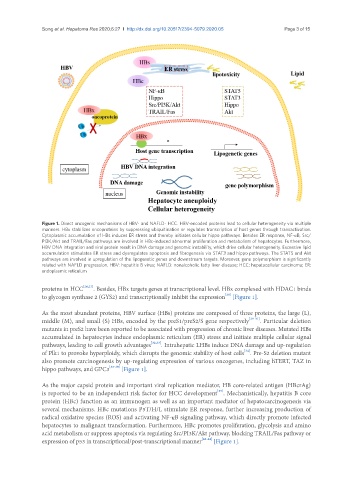
Figure 1. Direct oncogenic mechanisms of HBV- and NAFLD- HCC. HBV-encoded proteins lead to cellular heterogeneity via multiple
manners. HBx stabilizes oncoproteins by suppressing ubiquitination or regulates transcription of host genes through transactivation.
Cytoplasmic accumulation of HBs induces ER stress and thereby initiates cellular hippo pathways. Besides ER response, NF-κB, Src/
PI3K/Akt and TRAIL/Fas pathways are involved in HBc-induced abnormal proliferation and metabolism of hepatocytes. Furthermore,
HBV DNA integration and viral protein result in DNA damage and genomic instability, which drive cellular heterogeneity. Excessive lipid
accumulation stimulates ER stress and dysregulates apoptosis and fibrogenesis via STAT3 and hippo pathways. The STAT5 and Akt
pathways are involved in upregulation of the lipogenetic genes and downstream targets. Moreover, gene polymorphism is significantly
related with NAFLD progression. HBV: hepatitis B virus; NAFLD: nonalcoholic fatty liver disease; HCC: hepatocellular carcinoma; ER:
endoplasmic reticulum
proteins in HCC [26,27] . Besides, HBx targets genes at transcriptional level. HBx complexed with HDAC1 binds
[28]
to glycogen synthase 2 (GYS2) and transcriptionally inhibit the expression [Figure 1].
As the most abundant proteins, HBV surface (HBs) proteins are composed of three proteins, the large (L),
middle (M), and small (S) HBs, encoded by the preS1/preS2/S gene respectively [29-31] . Particular deletion
mutants in preS2 have been reported to be associated with progression of chronic liver diseases. Mutated HBs
accumulated in hepatocytes induce endoplasmic reticulum (ER) stress and initiate multiple cellular signal
pathways, leading to cell growth advantages [32,33] . Intrahepatic LHBs induce DNA damage and up-regulation
[34]
of Plk1 to provoke hyperploidy, which disrupts the genomic stability of host cells . Pre-S2 deletion mutant
also promote carcinogenesis by up-regulating expression of various oncogenes, including hTERT, TAZ in
hippo pathways, and GPC3 [35-38] [Figure 1].
As the major capsid protein and important viral replication mediator, HB core-related antigen (HBcrAg)
[39]
is reported to be an independent risk factor for HCC development . Mechanistically, hepatitis B core
protein (HBc) function as an immunogen as well as an important mediator of hepatocarcinogenesis via
several mechanisms. HBc mutations P5T/H/L stimulate ER response, further increasing production of
radical oxidative species (ROS) and activating NF-κB signaling pathway, which directly promote infected
hepatocytes to malignant transformation. Furthermore, HBc promotes proliferation, glycolysis and amino
acid metabolism or suppress apoptosis via regulating Src/PI3K/Akt pathway, blocking TRAIL/Fas pathway or
expression of p53 in transcriptional/post-transcriptional manner [40-44] [Figure 1].