Page 38 - Read Online
P. 38
Hafezi et al. Hepatoma Res 2020;6:23 I http://dx.doi.org/10.20517/2394-5079.2020.02 Page 3 of 7
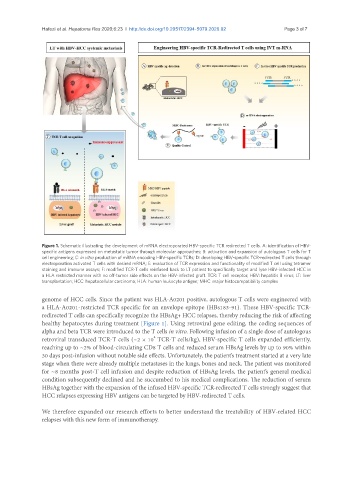
Figure 1. Schematic illustrating the development of mRNA electroporated HBV-specific TCR redirected T cells. A: identification of HBV-
specific antigens expressed on metastatic tumor through molecular approaches; B: activation and expansion of autologous T cells for T
cell engineering; C: in vitro production of mRNA encoding HBV-specific TCRs; D: developing HBV-specific TCR-redirected T cells through
electroporation activated T cells with desired mRNA; E: evaluation of TCR expression and functionality of modified T cell using tetramer
staining and immune assays; F: modified TCR-T cells reinfused back to LT patient to specifically target and lyse HBV-infected HCC in
a HLA restricted manner with no off-tumor side effects on the HBV-infected graft. TCR: T cell receptor; HBV: hepatitis B virus; LT: liver
transplantation; HCC: hepatocellular carcinoma; HLA: human leukocyte antigen; MHC: major histocompatibility complex
genome of HCC cells. Since the patient was HLA-A0201 positive, autologous T cells were engineered with
a HLA-A0201-restricted TCR specific for an envelope epitope (HBs183-91). These HBV-specific TCR-
redirected T cells can specifically recognize the HBsAg+ HCC relapses, thereby reducing the risk of affecting
healthy hepatocytes during treatment [Figure 1]. Using retroviral gene editing, the coding sequences of
alpha and beta TCR were introduced to the T cells in vitro. Following infusion of a single dose of autologous
4
retroviral transduced TCR-T cells (~2 × 10 TCR-T cells/kg), HBV-specific T cells expanded efficiently,
reaching up to ~2% of blood-circulating CD8 T cells and reduced serum HBsAg levels by up to 90% within
30 days post-infusion without notable side effects. Unfortunately, the patient’s treatment started at a very late
stage when there were already multiple metastases in the lungs, bones and neck. The patient was monitored
for ~8 months post-T cell infusion and despite reduction of HBsAg levels, the patient’s general medical
condition subsequently declined and he succumbed to his medical complications. The reduction of serum
HBsAg together with the expansion of the infused HBV-specific TCR-redirected T cells strongly suggest that
HCC relapses expressing HBV antigens can be targeted by HBV-redirected T cells.
We therefore expanded our research efforts to better understand the treatability of HBV-related HCC
relapses with this new form of immunotherapy.