Page 11 - Read Online
P. 11
Rutledge et al. Hepatoma Res 2019;5:31 I http://dx.doi.org/10.20517/2394-5079.2019.19 Page 5 of 12
n
n
n n
n n
n
n
n
n
n
n
n
n
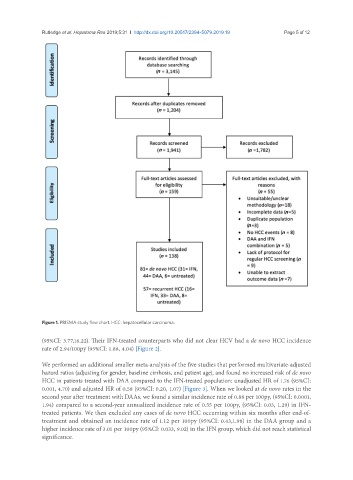
Figure 1. PRISMA study flow chart. HCC: hepatocellular carcinoma.
(95%CI: 3.77,16.22). Their IFN-treated counterparts who did not clear HCV had a de novo HCC incidence
rate of 2.94/100py (95%CI: 1.88, 4.04) [Figure 2].
We performed an additional smaller meta-analysis of the five studies that performed multivariate-adjusted
hazard ratios (adjusting for gender, baseline cirrhosis, and patient age), and found no increased risk of de novo
HCC in patients treated with DAA compared to the IFN-treated population: unadjusted HR of 1.76 (95%CI:
0.001, 4.70) and adjusted HR of 0.58 (95%CI: 0.20, 1.07) [Figure 3]. When we looked at de novo rates in the
second year after treatment with DAAs, we found a similar incidence rate of 0.88 per 100py, (95%CI: 0.0001,
1.94) compared to a second-year annualized incidence rate of 0.55 per 100py, (95%CI: 0.03, 1.29) in IFN-
treated patients. We then excluded any cases of de novo HCC occurring within six months after end-of-
treatment and obtained an incidence rate of 1.12 per 100py (95%CI: 0.43,1.98) in the DAA group and a
higher incidence rate of 3.01 per 100py (95%CI: 0.033, 9.02) in the IFN group, which did not reach statistical
significance.