Page 10 - Read Online
P. 10
Page 4 of 12 Rutledge et al. Hepatoma Res 2019;5:31 I http://dx.doi.org/10.20517/2394-5079.2019.19
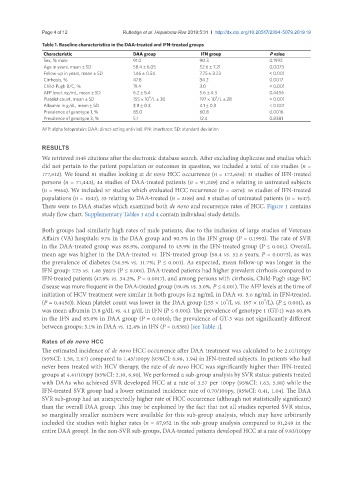
Table 1. Baseline characteristics in the DAA-treated and IFN-treated groups
Characteristic DAA group IFN group P value
Sex, % male 91.0 90.3 0.1992
Age in years, mean ± SD 58.4 ± 6.05 52.6 ± 7.21 0.0073
Follow-up in years, mean ± SD 1.46 ± 0.54 7.75 ± 3.23 < 0.001
Cirrhosis, % 47.8 34.2 0.0017
Child-Pugh B/C, % 19.4 3.0 < 0.001
AFP level, ng/mL, mean ± SD 6.2 ± 5.4 5.6 ± 4.3 0.4456
9
9
Platelet count, mean ± SD 155 × 10 /L ± 30 197 × 10 /L ± 28 < 0.001
Albumin in g/dL, mean ± SD 3.8 ± 0.3 4.1 ± 0.3 < 0.001
Prevalence of genotype 1, % 85.0 60.8 0.0016
Prevalence of genotype 3, % 5.1 12.4 0.8381
AFP: alpha fetoprotein; DAA: direct-acting antiviral; IFN: interferon; SD: standard deviation
RESULTS
We retrieved 3145 citations after the electronic database search. After excluding duplicates and studies which
did not pertain to the patient population or outcomes in question, we included a total of 138 studies (n =
177,512). We found 81 studies looking at de novo HCC occurrence (n = 172,636): 31 studies of IFN-treated
persons (n = 71,443), 44 studies of DAA-treated patients (n = 91,249) and 6 relating to untreated subjects
(n = 9944). We included 57 studies which evaluated HCC recurrence (n = 4876): 16 studies of IFN-treated
populations (n = 1043), 33 relating to DAA-treated (n = 2186) and 8 studies of untreated patients (n = 1647).
There were 16 DAA studies which examined both de novo and recurrence rates of HCC. Figure 1 contains
study flow chart. Supplementary Tables 3 and 4 contain individual study details.
Both groups had similarly high rates of male patients, due to the inclusion of large studies of Veterans
Affairs (VA) hospitals: 91% in the DAA group and 90.3% in the IFN group (P = 0.1992). The rate of SVR
in the DAA-treated group was 88.9%, compared to 45.9% in the IFN-treated group (P ≤ 0.001). Overall,
mean age was higher in the DAA-treated vs. IFN-treated group (58.4 vs. 52.6 years; P = 0.0073), as was
the prevalence of diabetes (34.5% vs. 11.7%; P ≤ 0.001). As expected, mean follow-up was longer in the
IFN group: 7.75 vs. 1.46 years (P ≤ 0.001). DAA-treated patients had higher prevalent cirrhosis compared to
IFN-treated patients (47.8% vs. 34.2%, P = 0.0017), and among persons with cirrhosis, Child-Pugh stage B/C
disease was more frequent in the DAA-treated group (19.4% vs. 3.0%, P ≤ 0.001). The AFP levels at the time of
initiation of HCV treatment were similar in both groups (6.2 ng/mL in DAA vs. 5.6 ng/mL in IFN-treated,
(P = 0.4456)). Mean platelet count was lower in the DAA group (155 × 10 /L vs. 197 × 10 /L), (P ≤ 0.001), as
9
9
was mean albumin (3.8 g/dL vs. 4.1 g/dL in IFN (P ≤ 0.001). The prevalence of genotype 1 (GT-1) was 60.8%
in the IFN and 85.0% in DAA group (P = 0.0016); the prevalence of GT-3 was not significantly different
between groups: 5.1% in DAA vs. 12.4% in IFN (P = 0.8381) [see Table 1].
Rates of de novo HCC
The estimated incidence of de novo HCC occurrence after DAA treatment was calculated to be 2.01/100py
(95%CI: 1.38, 2.67) compared to 1.45/100py (95%CI: 0.98, 1.94) in IFN-treated subjects. In patients who had
never been treated with HCV therapy, the rate of de novo HCC was significantly higher than IFN-treated
groups at 4.41/100py (95%CI: 2.10, 6.90). We performed a sub-group analysis by SVR status: patients treated
with DAAs who achieved SVR developed HCC at a rate of 3.57 per 100py (95%CI: 1.63, 5.88) while the
IFN-treated SVR group had a lower estimated incidence rate of 0.70/100py, (95%CI: 0.41, 1.04). The DAA
SVR sub-group had an unexpectedly higher rate of HCC occurrence (although not statistically significant)
than the overall DAA group. This may be explained by the fact that not all studies reported SVR status,
so marginally smaller numbers were available for this sub-group analysis, which may have arbitrarily
included the studies with higher rates (n = 87,952 in the sub-group analysis compared to 91,249 in the
entire DAA group). In the non-SVR sub-groups, DAA-treated patients developed HCC at a rate of 9.83/100py