Page 227 - Read Online
P. 227
Page 4 of 9 Lugaresi et al. Hepatoma Res 2018;4:67 I http://dx.doi.org/10.20517/2394-5079.2018.88
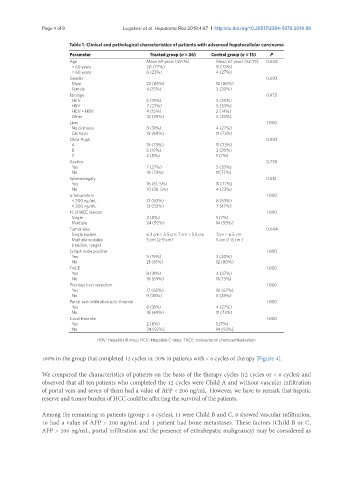
Table 1. Clinical and pathological characteristics of patients with advanced hepatocellular carcinoma
Parameter Treated group (n = 26) Control group (n = 15) P
Age Mean 69 years (49-76) Mean 67 years (52-75) 0.648
> 60 years 20 (77%) 11 (73%)
< 60 years 6 (23%) 4 (27%)
Gender 0.693
Male 22 (85%) 12 (80%)
Female 4 (15%) 3 (20%)
Etiology 0.972
HCV 5 (19%) 3 (20%)
HBV 7 (27%) 5 (33%)
HCV + HBV 4 (15%) 2 (14%)
Other 10 (39%) 5 (33%)
Liver 1.000
No cirrhosis 8 (31%) 4 (27%)
Cirrhosis 18 (69%) 11 (73%)
Child-Pugh 0.992
A 19 (73%) 11 (73%)
B 5 (19%) 3 (20%)
C 2 (8%) 1 (7%)
Ascites 0.730
Yes 7 (27%) 5 (33%)
No 19 (73%) 11(77%)
Splenomegaly 0.512
Yes 16 (61, 5%) 11 (77%)
No 10 (38, 5%) 4 (23%)
α-fetoprotein 1.000
< 200 ng/mL 13 (50%) 8 (53%)
> 200 ng/mL 13 (50%) 7 (47%)
N. of HCC lesions 1.000
Single 2 (8%) 1 (7%)
Multiple 24 (92%) 14 (93%)
Tumor size 0.644
Single nodule 6.3 cm × 5.5 cm; 7 cm × 5.5 cm 7cm × 6.5 cm
Multiple nodules 5 cm (2-9 cm) 5 cm (1-8 cm )
(median, range)
Lymph node positive 1.000
Yes 5 (19%) 3 (20%)
No 21 (81%) 12 (80%)
TACE 1.000
Yes 8 (31%) 4 (27%)
No 18 (69%) 11(73%)
Previous liver resection 1.000
Yes 17 (65%) 10 (67%)
No 9 (35%) 5 (33%)
Portal vein infiltration e/or thrombi 1.000
Yes 8 (31%) 4 (27%)
No 18 (69%) 11 (73%)
Caval thrombi 1.000
Yes 2 (8%) 1 (7%)
No 24 (92%) 14 (93%)
HBV: Hepatitis B virus; HCV: Hepatitis C virus; TACE: transarterial chemoembolization
100% in the group that completed 12 cycles vs. 20% in patients with < 6 cycles of therapy [Figure 4].
We compared the characteristics of patients on the basis of the therapy cycles (12 cycles or < 6 cycles) and
observed that all ten patients who completed the 12 cycles were Child A and without vascular infiltration
of portal vein and seven of them had a value of AFP < 200 ng/mL. However, we have to remark that hepatic
reserve and tumor burden of HCC could be affecting the survival of the patients.
Among the remaining 16 patients (group ≤ 6 cycles), 11 were Child B and C, 8 showed vascular infiltration,
10 had a value of AFP > 200 ng/mL and 1 patient had bone metastases. These factors (Child B or C,
AFP > 200 ng/mL, portal infiltration and the presence of extrahepatic malignancy) may be considered as