Page 93 - Read Online
P. 93
Papaluca et al. Hepatoma Res 2018;4:64 I http://dx.doi.org/10.20517/2394-5079.2018.53 Page 7 of 10
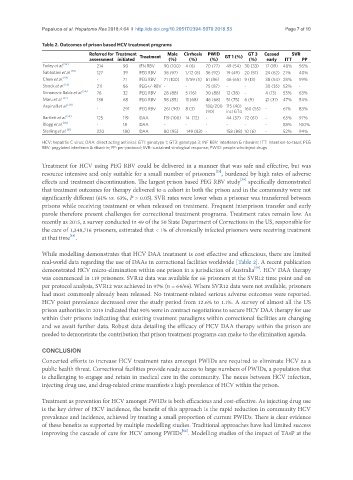
Table 2. Outcomes of prison based HCV treatment programs
Referred for Treatment Treatment Male Cirrhosis PWID GT 1 (%) GT 3 Ceased SVR
assessment initiated (%) (%) (%) (%) early ITT PP
Farley et al. [52] 214 90 IFN RBV 90 (100) 4 (6) 70 (77) 49 (54) 30 (33) 17 (19) 48% 56%
Sabbatani et al. [53] 127 39 PEG RBV 38 (97) 1/12 (8) 36 (92) 19 (49) 20 (51) 24 (62) 21% 40%
Chew et al. [54] - 71 PEG RBV 71 (100) 3/59 (5) 61 (86) 46 (65) 9 (13) 38 (54) 28% 59%
Strock et al. [55] 211 86 PEG+/-RBV - - 75 (87) - - 30 (35) 52% -
Simonovic Babic et al. [56] 76 32 PEG RBV 28 (88) 5 (16) 30 (88) 12 (38) - 4 (13) 53% 63%
Maru et al. [57] 138 68 PEG RBV 58 (85) 11 (68) 46 (68) 51 (75) 6 (9) 21 (31) 47% 54%
Aspinall et al. [58] 180/200 115 (40)
- 291 PEG RBV 261 (90) 8 (3) 160 (55) - 61% 83%
(90) incl GT4
Bartlett et al. [59] 125 119 DAA 119 (100) 14 (12) - 44 (37) 72 (61) - 65% 97%
Blogg et al. [60] - 18 DAA - - - - - - 83% 100%
Sterling et al. [61] 220 180 DAA 80 (95) 149 (83) - 158 (88) 10 (6) - 52% 94%
HCV: hepatitis C virus; DAA: direct acting antiviral; GT1: genotype 1; GT3: genotype 3; INF RBV: interferon & ribavirin; ITT: Intention-to-treat; PEG
RBV: pegylated interferon & ribavirin; PP: per-protocol; SVR: sustained virological response; PWID: people who inject drugs
Treatment for HCV using PEG RBV could be delivered in a manner that was safe and effective, but was
[25]
resource intensive and only suitable for a small number of prisoners , burdened by high rates of adverse
[58]
effects and treatment discontinuation. The largest prison based PEG RBV study specifically demonstrated
that treatment outcomes for therapy delivered to a cohort in both the prison and in the community were not
significantly different (61% vs. 63%, P > 0.05). SVR rates were lower when a prisoner was transferred between
prisons while receiving treatment or when released on treatment. Frequent interprison transfer and early
parole therefore present challenges for correctional treatment programs. Treatment rates remain low. As
recently as 2015, a survey conducted in 49 of the 50 State Department of Corrections in the US, responsible for
the care of 1,348,716 prisoners, estimated that < 1% of chronically infected prisoners were receiving treatment
[18]
at that time .
While modelling demonstrates that HCV DAA treatment is cost effective and efficacious, there are limited
real-world data regarding the use of DAAs in correctional facilities worldwide [Table 2]. A recent publication
[59]
demonstrated HCV micro-elimination within one prison in a jurisdiction of Australia . HCV DAA therapy
was commenced in 119 prisoners. SVR12 data was available for 66 prisoners at the SVR12 time point and on
per protocol analysis, SVR12 was achieved in 97% (n = 64/66). Where SVR12 data were not available, prisoners
had most commonly already been released. No treatment-related serious adverse outcomes were reported.
HCV point prevalence decreased over the study period from 12.6% to 1.1%. A survey of almost all the US
prison authorities in 2015 indicated that 90% were in contract negotiations to secure HCV DAA therapy for use
within their prisons indicating that existing treatment paradigms within correctional facilities are changing
and we await further data. Robust data detailing the efficacy of HCV DAA therapy within the prison are
needed to demonstrate the contribution that prison treatment programs can make to the elimination agenda.
CONCLUSION
Concerted efforts to increase HCV treatment rates amongst PWIDs are required to eliminate HCV as a
public health threat. Correctional facilities provide ready access to large numbers of PWIDs, a population that
is challenging to engage and retain in medical care in the community. The nexus between HCV infection,
injecting drug use, and drug-related crime manifests a high prevalence of HCV within the prison.
Treatment as prevention for HCV amongst PWIDs is both efficacious and cost-effective. As injecting drug use
is the key driver of HCV incidence, the benefit of this approach is the rapid reduction in community HCV
prevalence and incidence, achieved by treating a small proportion of current PWIDs. There is clear evidence
of these benefits as supported by multiple modelling studies. Traditional approaches have had limited success
improving the cascade of care for HCV among PWIDs . Modelling studies of the impact of TAsP at the
[62]