Page 45 - Read Online
P. 45
Page 8 of 14 Russo et al. Hepatoma Res 2018;4:25 I http://dx.doi.org/10.20517/2394-5079.2018.52
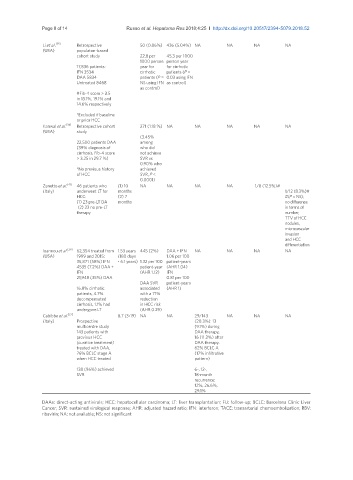
Li et al. [35] Retrospective 50 (0.86%) 436 (5.04%) NA NA NA NA
(USA) populaton-based
cohort study 22.8 per 45.3 per 1000
1000 person person year
17,836 patients: year for for cirrhotic
IFN 3534 cirrhotic patients (P =
DAA 5834 patients (P = 0.03 using IFN
Untreated 8468 NS using IFN as control)
as control)
#Fib-4 score > 3.5
in 13.1%, 19.1% and
14.6% respectively
*Excluded if baseline
or prior HCC
Kanwal et al. [34] Retrospective cohort 271 (1.18 %) NA NA NA NA NA
(USA) study
(3.45%
22,500 patients DAA among
(39% diagnosis of who did
cirrhosis, Fib-4 score not achieve
> 3.25 in 29.7 %) SVR vs.
0.90% who
*No previous history achieved
of HCC SVR, P <
0.0001)
Zanetto et al. [41] 46 patients who (1) 10 NA NA NA NA 1/8 (12.5%)#
(Italy) underwent LT for months 1/12 (8.3%)#
HCC: (2) 7 #(P = NS);
(1) 23 pre-LT DA months no difference
(2) 23 no pre-LT in terms of
therapy number,
TTV of HCC
nodules,
microvascular
invasion
and HCC
differentiation
Ioannou et al. [29] 62,354 treated from 1.53 years 445 (2%) DAA + IFN NA NA NA NA
(USA) 1999 and 2015: (180 days 1.06 per 100
35,871 (58%) IFN - 6.1 years) 1.32 per 100 patient-years
4535 (7.2%) DAA + patient-year (AHR 1.04)
IFN (AHR 1.12) IFN
21,948 (35%) DAA 0.81 per 100
DAA SVR patient-years
16.8% cirrhotic associated (AHR 1)
patients, 4.7% with a 71%
decompensated reduction
cirrhosis, 1.1% had in HCC risk
undergone LT (AHR 0.29)
Cabibbo et al. [27] 8.7 (3-19) NA NA 29/143 NA NA NA
(Italy) Prospective (20.3%): 13
multicentre study (9.1%) during
143 patients with DAA therapy,
previous HCC 16 (11.2%) after
(curative treatment) DAA therapy.
treated with DAA, 62% BCLC A
76% BCLC stage A (17% infiltrative
when HCC treated pattern)
138 (96%) achieved 6-, 12-,
SVR 18-month
recurrence:
12%, 26.6%,
29.1%
DAAs: direct-acting antivirals; HCC: hepatocellular carcinoma; LT: liver transplantation; FU: follow-up; BCLC: Barcelona Clinic Liver
Cancer; SVR: sustained virological response; AHR: adjusted hazard ratio; IFN: interferon; TACE: transarterial chemoembolization; RBV:
ribavirin; NA: not available; NS: not significant