Page 138 - Read Online
P. 138
Page 4 Cai et al. Extracell Vesicles Circ Nucleic Acids 2023;4:262-82 https://dx.doi.org/10.20517/evcna.2023.10
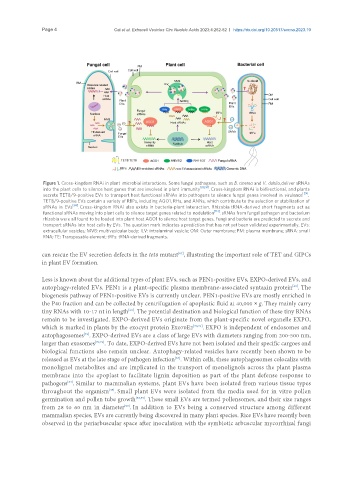
Figure 1. Cross-kingdom RNAi in plant–microbial interactions. Some fungal pathogens, such as B. cinerea and V. dahlia, deliver sRNAs
into the plant cells to silence host genes that are involved in plant immunity [88,91] . Cross-kingdom RNAi is bidirectional, and plants
secrete TET8/9-positive EVs to transport host functional sRNAs into pathogens to silence fungal genes involved in virulence [29] .
TET8/9-positive EVs contain a variety of RBPs, including AGO1, RHs, and ANNs, which contribute to the selection or stabilization of
sRNAs in EVs [56] . Cross-kingdom RNAi also exists in bacteria-plant interaction. Rhizobia tRNA-derived short fragments act as
functional sRNAs moving into plant cells to silence target genes related to nodulation [113] . sRNAs from fungal pathogen and bacterium
rhizobia were all found to be loaded into plant host AGO1 to silence host target genes. Fungi and bacteria are predicted to secrete and
transport sRNAs into host cells by EVs. The question mark indicates a prediction that has not yet been validated experimentally. EVs:
extracellular vesicles; MVB: multivesicular body; ILV: intraluminal vesicle; OM: Outer membrane; PM: plasma membrane; sRNA: small
RNA; TE: Transposable element; tRFs: tRNA-derived fragments.
[67]
can rescue the EV secretion defects in the tet8 mutant , illustrating the important role of TET and GIPCs
in plant EV formation.
Less is known about the additional types of plant EVs, such as PEN1-positive EVs, EXPO-derived EVs, and
autophagy-related EVs. PEN1 is a plant-specific plasma membrane-associated syntaxin protein . The
[69]
biogenesis pathway of PEN1-positive EVs is currently unclear. PEN1-positive EVs are mostly enriched in
the P40 fraction and can be collected by centrifugation of apoplastic fluid at 40,000 × g. They mainly carry
tiny RNAs with 10-17 nt in length . The potential destination and biological function of these tiny RNAs
[63]
remain to be investigated. EXPO-derived EVs originate from the plant-specific novel organelle EXPO,
which is marked in plants by the exocyst protein Exo70E2 [70,71] . EXPO is independent of endosomes and
autophagosomes . EXPO-derived EVs are a class of large EVs with diameters ranging from 200-500 nm,
[71]
larger than exosomes [70,71] . To date, EXPO-derived EVs have not been isolated and their specific cargoes and
biological functions also remain unclear. Autophagy-related vesicles have recently been shown to be
released as EVs at the late stage of pathogen infection . Within cells, these autophagosomes colocalize with
[64]
monolignol metabolites and are implicated in the transport of monolignols across the plant plasma
membrane into the apoplast to facilitate lignin deposition as part of the plant defense response to
[64]
pathogens . Similar to mammalian systems, plant EVs have been isolated from various tissue types
[45]
throughout the organism . Small plant EVs were isolated from the media used for in vitro pollen
germination and pollen tube growth [58,59] . These small EVs are termed pollensomes, and their size ranges
from 28 to 60 nm in diameter . In addition to EVs being a conserved structure among different
[59]
mammalian species, EVs are currently being discovered in many plant species. Rice EVs have recently been
observed in the periarbuscular space after inoculation with the symbiotic arbuscular mycorrhizal fungi