Page 104 - Read Online
P. 104
Liu et al. Chem Synth 2023;3:11 https://dx.doi.org/10.20517/cs.2022.46 Page 5 of 8
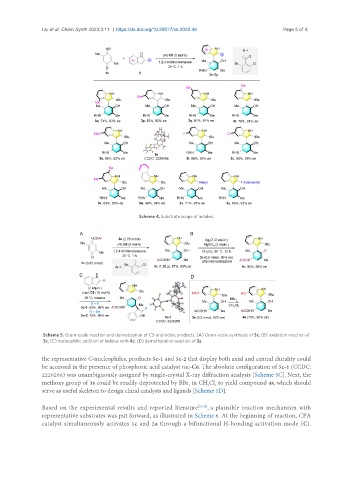
Scheme 4. Substrate scope of indoles.
Scheme 5. Gram-scale reaction and derivatization of C3-arylindole products. (A) Gram-scale synthesis of 3c; (B) oxidation reaction of
3c; (C) nucleophilic addition of indoles with 4c; (D) demethylation reaction of 3s.
the representative C-nucleophiles, products 5c-1 and 5c-2 that display both axial and central chirality could
be accessed in the presence of phosphoric acid catalyst rac-C6. The absolute configuration of 5c-1 (CCDC:
2226266) was unambiguously assigned by single-crystal X-ray diffraction analysis [Scheme 5C]. Next, the
methoxy group of 3s could be readily deprotected by BBr in CH Cl to yield compound 4s, which should
2
3
2
serve as useful skeleton to design chiral catalysts and ligands [Scheme 5D].
Based on the experimental results and reported literature [23,24] , a plausible reaction mechanism with
representative substrates was put forward, as illustrated in Scheme 6. At the beginning of reaction, CPA
catalyst simultaneously activates 1c and 2a through a bifunctional H-bonding activation mode (C).