Page 62 - Read Online
P. 62
Page 752 Lyons et al. Cancer Drug Resist 2021;4:745-54 https://dx.doi.org/10.20517/cdr.2021.37
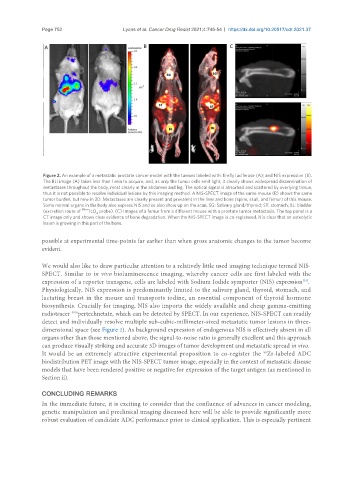
Figure 2. An example of a metastatic prostate cancer model with the tumors labeled with: firefly luciferase (A); and NIS expression (B).
The BLI image (A) takes less than 1 min to acquire, and, as only the tumor cells emit light, it clearly shows widespread dissemination of
metastases throughout the body, most clearly in the abdomen and leg. The optical signal is absorbed and scattered by overlying tissue,
thus it is not possible to resolve individual lesions by this imaging method. A NIS-SPECT image of the same mouse (B) shows the same
tumor burden, but now in 3D. Metastases are clearly present and prevalent in the liver and bone (spine, skull, and femur) of this mouse.
Some normal organs in the body also express NIS and so also show up on the scan. SG: Salivary gland/thyroid; ST: stomach; BL: bladder
(excretion route of 99m TcO probe). (C) Images of a femur from a different mouse with a prostate tumor metastasis. The top panel is a
4
CT image only and shows clear evidence of bone degradation. When the NIS-SPECT image is co-registered, it is clear that an osteolytic
lesion is growing in this part of the bone.
possible at experimental time-points far earlier than when gross anatomic changes to the tumor become
evident.
We would also like to draw particular attention to a relatively little used imaging technique termed NIS-
SPECT. Similar to in vivo bioluminescence imaging, whereby cancer cells are first labeled with the
[33]
expression of a reporter transgene, cells are labeled with Sodium Iodide symporter (NIS) expression .
Physiologically, NIS expression is predominantly limited to the salivary gland, thyroid, stomach, and
lactating breast in the mouse and transports iodine, an essential component of thyroid hormone
biosynthesis. Crucially for imaging, NIS also imports the widely available and cheap gamma-emitting
radiotracer pertechnetate, which can be detected by SPECT. In our experience, NIS-SPECT can readily
99m
detect and individually resolve multiple sub-cubic-millimeter-sized metastatic tumor lesions in three-
dimensional space (see Figure 2). As background expression of endogenous NIS is effectively absent in all
organs other than those mentioned above, the signal-to-noise ratio is generally excellent and this approach
can produce visually striking and accurate 3D images of tumor development and metastatic spread in vivo.
It would be an extremely attractive experimental proposition to co-register the Zr-labeled ADC
89
biodistribution PET image with the NIS-SPECT tumor image, especially in the context of metastatic disease
models that have been rendered positive or negative for expression of the target antigen (as mentioned in
Section ii).
CONCLUDING REMARKS
In the immediate future, it is exciting to consider that the confluence of advances in cancer modeling,
genetic manipulation and preclinical imaging discussed here will be able to provide significantly more
robust evaluation of candidate ADC performance prior to clinical application. This is especially pertinent