Page 58 - Read Online
P. 58
Page 748 Lyons et al. Cancer Drug Resist 2021;4:745-54 https://dx.doi.org/10.20517/cdr.2021.37
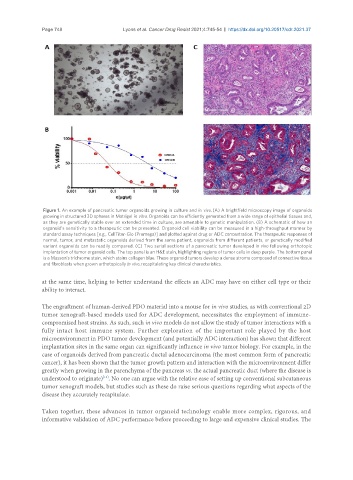
Figure 1. An example of pancreatic tumor organoids growing in culture and in vivo. (A) A brightfield microscopy image of organoids
growing in structured 3D spheres in Matrigel in vitro. Organoids can be efficiently generated from a wide range of epithelial tissues and,
as they are genetically stable over an extended time in culture, are amenable to genetic manipulation. (B) A schematic of how an
organoid’s sensitivity to a therapeutic can be presented. Organoid cell viability can be measured in a high-throughput manner by
standard assay techniques [e.g., CellTiter-Glo (Promega)] and plotted against drug or ADC concentration. The therapeutic responses of
normal, tumor, and metastatic organoids derived from the same patient, organoids from different patients, or genetically modified
variant organoids can be readily compared. (C) Two serial sections of a pancreatic tumor developed in vivo following orthotopic
implantation of tumor organoid cells. The top panel is an H&E stain, highlighting regions of tumor cells in deep purple. The bottom panel
is a Masson’s trichrome stain, which stains collagen blue. These organoid tumors develop a dense stroma composed of connective tissue
and fibroblasts when grown orthotopically in vivo, recapitulating key clinical characteristics.
at the same time, helping to better understand the effects an ADC may have on either cell type or their
ability to interact.
The engraftment of human-derived PDO material into a mouse for in vivo studies, as with conventional 2D
tumor xenograft-based models used for ADC development, necessitates the employment of immune-
compromised host strains. As such, such in vivo models do not allow the study of tumor interactions with a
fully intact host immune system. Further exploration of the important role played by the host
microenvironment in PDO tumor development (and potentially ADC interaction) has shown that different
implantation sites in the same organ can significantly influence in vivo tumor biology. For example, in the
case of organoids derived from pancreatic ductal adenocarcinoma (the most common form of pancreatic
cancer), it has been shown that the tumor growth pattern and interaction with the microenvironment differ
greatly when growing in the parenchyma of the pancreas vs. the actual pancreatic duct (where the disease is
understood to originate) . No one can argue with the relative ease of setting up conventional subcutaneous
[15]
tumor xenograft models, but studies such as these do raise serious questions regarding what aspects of the
disease they accurately recapitulate.
Taken together, these advances in tumor organoid technology enable more complex, rigorous, and
informative validation of ADC performance before proceeding to large and expensive clinical studies. The