Page 52 - Read Online
P. 52
Giuliani et al. Cancer Drug Resist 2021;4:740-4 https://dx.doi.org/10.20517/cdr.2021.14 Page 742
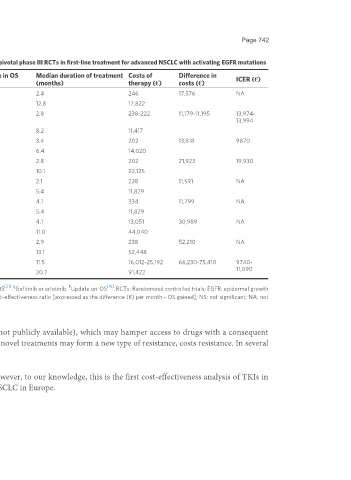
Table 2. Pharmacological costs and difference in OS with the different treatment regimens of the pivotal phase III RCTs in first-line treatment for advanced NSCLC with activating EGFR mutations
N of OS P- Difference in OS Median duration of treatment Costs of Difference in
Ref./Trial Comparative regimens ICER (€)
patients (months) value (months) (months) therapy (€) costs (€)
[6] a a
Zhou et al. Carboplatin + gemcitabine 72 27.2 NS -4.4 2.4 246 17,576 NA
OPTIMAL a
Erlotinib 82 22.8 12.8 17,822
[7] b b
Rosell et al. Cisplatin + 87 22.1 NS 0.8 2.8 238-222 11,179-11,195 13,974-
EURTAC docetaxel/gemcitabine 13,994
b
Erlotinib 86 22.9 8.2 11,417
[8] c c
Mok et al. Carboplatin + paclitaxel 129 17.4 NS 1.4 3.4 202 13,818 9870
IPASS c
Gefitinib 132 18.8 6.4 14,020
[9] d d
Maemondo et al. Carboplatin + paclitaxel 110 26.6 NS 1.1 2.8 202 21,923 19,930
NEJ2002 d
Gefitinib 114 27.7 10.1 22,125
[10] e e
Mitsudomi et al. Cisplatin + docetaxel 86 37.3 NS -2.4 2.1 238 11,591 NA
WJTOG3405 e
Gefitinib 86 34.9 5.4 11,829
[11]
Han et al. Cisplatin + gemcitabine 26 22.9 NS -0.6 4.1 334 11,799 NA
First-SIGNAL
Gefitinib 22 22.3 5.4 11,829
[12] f f
Sequist et al. Cisplatin + pemetrexed 111 28.2 NS 0.0 4.1 13,051 30,989 NA
LUX-Lung 3 f
Afatinib 229 28.2 11.0 44,040
[13] f f
Wu et al. Cisplatin + gemcitabine 122 23.5 NS -0.4 2.9 238 52,210 NA
LUX-Lung 6 f
Afatinib 242 23.1 13.1 52,448
[14] g h h
Soria et al. Standard EGFR-TKI 277 31.8 0.046 6.8 11.5 16,012-25,192 66,230-75,410 9740-
FLAURA h 11,090
Osimertinib 279 38.6 20.7 91,422
a [17] b [18] c [15] d [19] e [20] f [21] g h [16]
Update on OS . Update on OS . Update on OS . Update on OS . Update on OS . Update on OS . Gefitinib or erlotinib. Update on OS . RCTs: Randomized controlled trials; EGFR: epidermal growth
factor receptor; NSCLC: non-small cell lung cancer; N: number; OS: overall survival; ICER: incremental cost-effectiveness ratio [expressed as the difference (€) per month - OS gained]; NS: not significant; NA: not
applicable.
allow more patients to be treated. This results in “confidential rebates” (i.e., not publicly available), which may hamper access to drugs with a consequent
overpayment without improving the value of drugs. The extraordinary costs of novel treatments may form a new type of resistance, costs resistance. In several
countries, this may preclude treatments with these compounds.
There are several published articles, mostly in China, regarding this topic. However, to our knowledge, this is the first cost-effectiveness analysis of TKIs in
patients with activating EGFR-mutations in first-line treatment for advanced NSCLC in Europe.