Page 25 - Read Online
P. 25
Page 4 Conroy et al. Cancer Drug Resist 2021;4:543-58 https://dx.doi.org/10.20517/cdr.2021.07
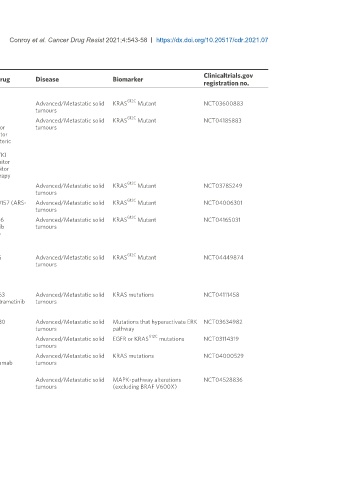
Table 1. Ongoing RAS inhibitor trials
Clinicaltrials.gov
Study/Phase Drug Disease Biomarker
registration no.
Direct RAS inhibitors
G12C
A phase I/II, study evaluating the safety, tolerability, PK, and efficacy of AMG 510 in subjects AMG510 Advanced/Metastatic solid KRAS Mutant NCT03600883
with solid tumours with a specific KRAS mutation (CodeBreak 100) tumours
G12C
A Phase 1b, Protocol Evaluating the Safety, Tolerability, Pharmacokinetics, and Efficacy of AMG510 Advanced/Metastatic solid KRAS Mutant NCT04185883
AMG 510 (pINN) Sotorasib Monotherapy and in Combination with Other Anti-cancer PD1 inhibitor tumours
Therapies in Subjects with Advanced Solid Tumours With KRAS p.G12C Mutation (CodeBreak MEK inhibitor
101) SHP2 allosteric
inhibitor
Pan-ErbB TKI
PD-L1 inhibitor
EGFR inhibitor
Chemotherapy
G12C
Phase I/II Study of MRTX849 in patients having a KRAS G12C Mutation KRYSTAL-1 MRTX849 Advanced/Metastatic solid KRAS Mutant NCT03785249
tumours
G12C
First-in-Human Study of JNJ-74699157 in participants with Tumours Harboring the KRAS JNJ-74699157 (ARS- Advanced/Metastatic solid KRAS Mutant NCT04006301
G12C Mutation 3248) tumours
G12C
A Phase I/II study of LY3499446 administered to patients with advanced solid tumours with LY3499446 Advanced/Metastatic solid KRAS Mutant NCT04165031
KRAS G12C mutation Abemaciclib tumours
Cetuximab
Erlotinib
docetaxel
G12C
A Study to evaluate the safety, Pharmacokinetics, and activity of GDC-6036 in participants GDC-6036 Advanced/Metastatic solid KRAS Mutant NCT04449874
with advanced or metastatic solid tumours with a KRAS G12C mutation tumours
Indirect Targeting of RAS
SOS inhibitor
A study to test different doses of BI-1701963 alone and in combined with trametinib in patients BI-1701963 Advanced/Metastatic solid KRAS mutations NCT04111458
with different types of advanced cancer (solid tumours with KRAS mutation) BI-3406 trametinib tumours
SHP2 inhibitors
Dose escalation of RMC-4630 monotherapy in relapsed/refractory solid tumours RMC-4630 Advanced/Metastatic solid Mutations that hyperactivate ERK NCT03634982
tumours pathway
G12C
Dose finding study of TNO155 in adult patients with advanced solid tumours TNO155 Advanced/Metastatic solid EGFR or KRAS mutations NCT03114319
tumours
Phase Ib study of TNO155 in combination with Spartalizumab or Ribociclib in selected TNO155 Advanced/Metastatic solid KRAS mutations NCT04000529
malignancies Spartalizumab tumours
ribociclib
First-in-Human Study of the SHP2 Inhibitor BBP-398 in patients with Advanced Solid Tumours BBP-398 Advanced/Metastatic solid MAPK-pathway alterations NCT04528836
tumours (excluding BRAF V600X)
Farnesyltransferase inhibitors