Page 43 - Read Online
P. 43
Page 994 Juhlin. Cancer Drug Resist 2020;3:992-1000 I http://dx.doi.org/10.20517/cdr.2020.66
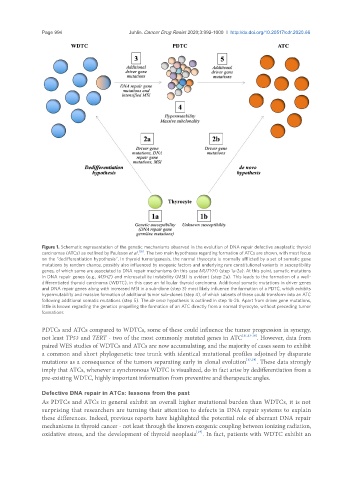
Figure 1. Schematic representation of the genetic mechanisms observed in the evolution of DNA repair defective anaplastic thyroid
carcinomas (ATCs) as outlined by Paulsson et al. [26] . The two main hypotheses regarding formation of ATCs are shown, with most focus
on the “dedifferentiation hypothesis”. In thyroid tumorigenesis, the normal thyrocyte is normally afflicted by a set of somatic gene
mutations by random chance, possibly also influenced by exogenic factors and underlying rare constitutional variants in susceptibility
genes, of which some are associated to DNA repair mechanisms (in this case MUTYH) (step 1a-2a). At this point, somatic mutations
in DNA repair genes (e.g., MSH2) and microsatellite instability (MSI) is evident (step 2a). This leads to the formation of a well-
differentiated thyroid carcinoma (WDTC), in this case an follicular thyroid carcinoma. Additional somatic mutations in driver genes
and DNA repair genes along with increased MSI in a sub-clone (step 3) most likely influence the formation of a PDTC, which exhibits
hypermutability and massive formation of additional tumor sub-clones (step 4), of which subsets of these could transform into an ATC
following additional somatic mutations (step 5). The de novo hypothesis is outlined in step 1b-2b. Apart from driver gene mutations,
little is known regarding the genetics propelling the formation of an ATC directly from a normal thyrocyte, without preceding tumor
formations
PDTCs and ATCs compared to WDTCs, some of these could influence the tumor progression in synergy,
not least TP53 and TERT - two of the most commonly mutated genes in ATC [11,13-16] . However, data from
paired WES studies of WDTCs and ATCs are now accumulating, and the majority of cases seem to exhibit
a common and short phylogenetic tree trunk with identical mutational profiles adjoined by disparate
mutations as a consequence of the tumors separating early in clonal evolution [17,18] . These data strongly
imply that ATCs, whenever a synchronous WDTC is visualized, do in fact arise by dedifferentiation from a
pre-existing WDTC, highly important information from preventive and therapeutic angles.
Defective DNA repair in ATCs: lessons from the past
As PDTCs and ATCs in general exhibit an overall higher mutational burden than WDTCs, it is not
surprising that researchers are turning their attention to defects in DNA repair systems to explain
these differences. Indeed, previous reports have highlighted the potential role of aberrant DNA repair
mechanisms in thyroid cancer - not least through the known exogenic coupling between ionizing radiation,
[19]
oxidative stress, and the development of thyroid neoplasia . In fact, patients with WDTC exhibit an