Page 16 - Read Online
P. 16
Page 998 Gmeiner. Cancer Drug Resist 2019;2:994-1001 I http://dx.doi.org/10.20517/cdr.2019.95
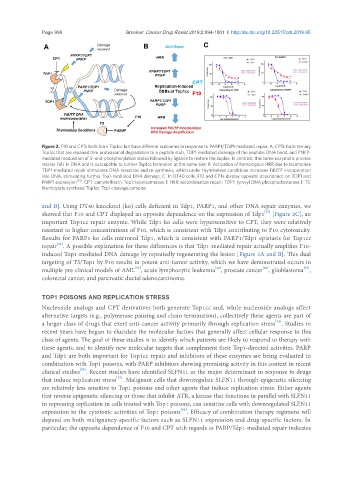
Figure 2. F10 and CPTs both form Top1cc but have different outcomes in response to PARP1/TDP1-mediated repair. A: CPTs form ternary
Top1cc that are repaired thru proteasomal degradation to a peptide stub, TDP1-mediated cleavage of the peptide: DNA bond, and PNKP-
mediated modulation of 5’-end-phosphorylation status followed by ligation to restore the duplex. In contrast, this same enzymatic process
retains FdU in DNA and is susceptible to further Top1cc formation at the same site; B: Activation of homologous HRR due to incomplete
TDP1-mediated repair stimulates DNA resection and re-synthesis, which under thymineless conditions increases FdUTP incorporation
into DNA, stimulating further Top1-mediated DNA damage; C: In DT40 cells, F10 and CPTs display opposite dependence on TDP1 and
PARP1 expression [53] . CPT: camptothecin; Top1: topoisomerase 1; HRR: recombination repair; TDP1: tyrosyl DNA phosphodiesterase 1; TS:
thymidylate synthase; Top1cc: Top1 cleavage complex
and B]. Using DT40 knockout (ko) cells deficient in Tdp1, PARP1, and other DNA repair enzymes, we
[53]
showed that F10 and CPT displayed an opposite dependence on the expression of Tdp1 [Figure 2C], an
important Top1cc repair enzyme. While Tdp1-ko cells were hypersensitive to CPT, they were relatively
resistant to higher concentrations of F10, which is consistent with Tdp1 contributing to F10 cytotoxicity.
Results for PARP1-ko cells mirrored Tdp1, which is consistent with PARP1/Tdp1 epistasis for Top1cc
[54]
repair . A possible explanation for these differences is that Tdp1-mediated repair actually amplifies F10-
induced Top1-mediated DNA damage by repeatedly regenerating the lesion [Figure 2A and B]. This dual
targeting of TS/Top1 by F10 results in potent anti-tumor activity, which we have demonstrated occurs in
[56]
[55]
[48]
[57]
multiple pre-clinical models of AML , acute lymphocytic leukemia , prostate cancer , glioblastoma ,
colorectal cancer, and pancreatic ductal adenocarcinoma.
TOP1 POISONS AND REPLICATION STRESS
Nucleoside analogs and CPT derivatives both generate Top1cc and, while nucleoside analogs affect
alternative targets (e.g., polymerase pausing and chain termination), collectively these agents are part of
[31]
a larger class of drugs that exert anti-cancer activity primarily through replication stress . Studies in
recent years have begun to elucidate the molecular factors that generally affect cellular response to this
class of agents. The goal of these studies is to identify which patients are likely to respond to therapy with
these agents, and to identify new molecular targets that complement their Top1-directed activities. PARP
and Tdp1 are both important for Top1cc repair and inhibitors of these enzymes are being evaluated in
combination with Top1 poisons, with PARP inhibitors showing promising activity in this context in recent
[58]
clinical studies . Recent studies have identified SLFN11 as the major determinant in response to drugs
[33]
that induce replication stress . Malignant cells that downregulate SLFN11 through epigenetic silencing
are relatively less sensitive to Top1 poisons and other agents that induce replication stress. Either agents
that reverse epigenetic silencing or those that inhibit ATR, a kinase that functions in parallel with SLFN11
in repressing replication in cells treated with Top1 poisons, can sensitize cells with downregulated SLFN11
[59]
expression to the cytotoxic activities of Top1 poisons . Efficacy of combination therapy regimens will
depend on both malignancy-specific factors such as SLFN11 expression and drug-specific factors. In
particular, the opposite dependence of F10 and CPT with regards to PARP/Tdp1-mediated repair indicates