Page 67 - Read Online
P. 67
Meyer et al. Cancer Drug Resist 2019;2:313-25 I http://dx.doi.org/10.20517/cdr.2019.11 Page 321
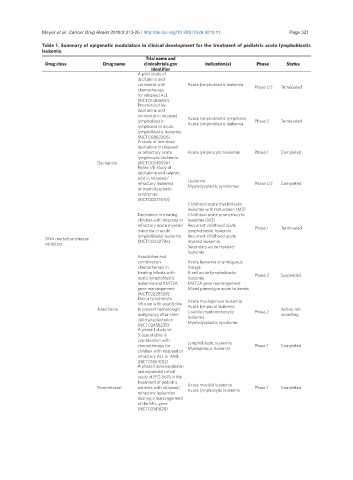
Table 1. Summary of epigenetic modulators in clinical development for the treatment of pediatric acute lymphoblastic
leukemia
Trial name and
Drug class Drug name clinicaltrials.gov Indication(s) Phase Status
identifier
A pilot study of
decitabine and
vorinostat with Acute lymphoblastic leukemia Phase 1/2 Terminated
chemotherapy
for relapsed ALL
(NCT01483690)
Pre-reinductive
decitabine and
vorinostat in relapsed
lymphoblastic Acute lymphoblastic lymphoma Phase 2 Terminated
lymphoma or acute Acute lymphoblastic leukemia
lymphoblastic leukemia
(NCT00882206)
A study of low-dose
decitabine in relapsed
or refractory acute Acute lymphocytic leukemia Phase 1 Completed
lymphocytic leukemia
Decitabine (NCT00349596)
Phase I/II study of
decitabine and valproic
acid in relapsed/
refractory leukemia Leukemia Phase 1/2 Completed
or myelodysplastic Myelodysplastic syndromes
syndromes
(NCT00075010)
Childhood acute myeloblastic
leukemia with maturation (M2)
Decitabine in treating Childhood acute promyelocytic
children with relapsed or leukemia (M3)
refractory acute myeloid Recurrent childhood acute Phase 1 Terminated
leukemia or acute lymphoblastic leukemia
lymphoblastic leukemia Recurrent childhood acute
DNA methyltransferase
inhibitors (NCT00042796) myeloid leukemia
Secondary acute myeloid
leukemia
Azacitidine and
combination Acute leukemia or ambiguous
chemotherapy in lineage
treating infants with B-cell acute lymphoblastic
acute lymphoblastic leukemia Phase 2 Suspended
leukemia and KMT2A KMT2A gene rearrangement
gene rearrangement Mixed phenotype acute leukemia
(NCT02828358)
Donor lymphocyte Acute myelogenous leukemia
infusion with azacitidine Acute lymphoid leukemia
Azacitidine to prevent hematologic Juvenile myelomonocytic Phase 2 Active, not
malignancy after stem leukemia recruiting
cell transplantation Myelodysplastic syndrome
(NCT02458235)
A phase I study of
5-azacytidine in
combination with
chemotherapy for Lymphoblastic leukemia Phase 1 Completed
children with relapsed or Myelogenous leukemia
refractory ALL or AML
(NCT01861002)
A phase I dose escalation
and expanded cohort
study of EPZ-5676 in the
treatment of pediatric Acute myeloid leukemia
Pinometostat patients with relapsed/ Acute lymphocytic leukemia Phase 1 Completed
refractory leukemias
bearing a rearrangement
of the MLL gene
(NCT02141828)