Page 34 - Read Online
P. 34
Page 222 Peixoto et al. Cancer Drug Resist 2018;1:219-29 I http://dx.doi.org/10.20517/cdr.2018.17
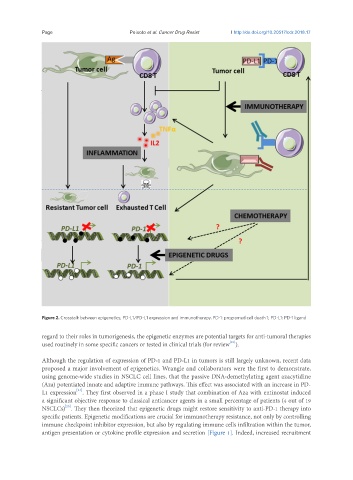
Figure 2. Crosstalk between epigenetics, PD-L1/PD-L1 expression and immunotherapy. PD-1: programed cell death 1; PD-L1: PD-1 ligand
regard to their roles in tumorigenesis, the epigenetic enzymes are potential targets for anti-tumoral therapies
[23]
used routinely in some specific cancers or tested in clinical trials (for review ).
Although the regulation of expression of PD-1 and PD-L1 in tumors is still largely unknown, recent data
proposed a major involvement of epigenetics. Wrangle and collaborators were the first to demonstrate,
using genome-wide studies in NSCLC cell lines, that the passive DNA-demethylating agent azacytidine
(Aza) potentiated innate and adaptive immune pathways. This effect was associated with an increase in PD-
[24]
L1 expression . They first observed in a phase I study that combination of Aza with entinostat induced
a significant objective response to classical anticancer agents in a small percentage of patients (4 out of 19
[25]
NSCLCs) . They then theorized that epigenetic drugs might restore sensitivity to anti-PD-1 therapy into
specific patients. Epigenetic modifications are crucial for immunotherapy resistance, not only by controlling
immune checkpoint inhibitor expression, but also by regulating immune cells infiltration within the tumor,
antigen presentation or cytokine profile expression and secretion [Figure 1]. Indeed, increased recruitment