Page 75 - Read Online
P. 75
Tucker et al. Cancer Drug Resist 2019;2:803-12 I http://dx.doi.org/10.20517/cdr.2019.09 Page 805
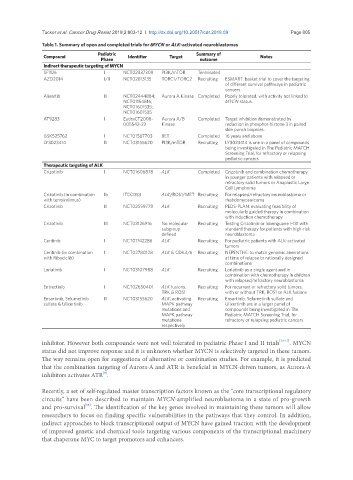
Table 1. Summary of open and completed trials for MYCN or ALK-activated neuroblastomas
Compound Pediatric Identifier Target Summary of Notes
Phase outcome
Indirect therapeutic targeting of MYCN
SF1126 I NCT02337309 PI3K/mTOR Terminated
AZD2014 I/II NCT02813135 TORC1/TORC2 Recruiting ESMART: basket trial to cover the targeting
of different survival pathways in pediatric
cancers
Alisertib II NCT02444884; Aurora A Kinase Completed Poorly tolerated, with activity not linked to
NCT01154816; MYCN status.
NCT01601535;
NCT01601535
AT9283 I EudraCT2008- Aurora A/B Completed Target inhibition demonstrated by
005542-23 Kinase reduction in phosphor-histone 3 in paired
skin punch biopsies.
GSK525762 I NCT01587703 BET Completed 16 years and above
LY3023414 II NCT03155620 PI3K/mTOR Recruiting LY3023414 is one in a panel of compounds
being investigated in The Pediatric MATCH
Screening Trial, for refractory or relapsing
pediatric cancers
Therapeutic targeting of ALK
Crizotinib I NCT01606878 ALK Completed Crizotinib and combination chemotherapy
in younger patients with relapsed or
refractory solid tumors or Anaplastic Large
Cell Lymphoma
Crizotinib (in combination Ib ITCC053 ALK/ROS1/MET Recruiting For relapsed/refractory neuroblastoma or
with temsirolimus) rhabdomyosarcoma
Crizotinib II NCT02559778 ALK Recruiting PEDS-PLAN: evaluating feasibility of
molecularly guided therapy in combination
with induction chemotherapy
Crizotinib III NCT03126916 No molecular Recruiting Testing Crizotinib or Iobenguane I-131 with
subgroup standard therapy for patients with high-risk
defined neuroblastoma
Ceritinib I NCT01742286 ALK Recruiting For pediatric patients with ALK-activated
tumors
Ceritinib (in combination I NCT02780128 ALK & CDK4/6 Recruiting NEPENTHE: to match genomic aberrations
with Ribociclib) at time of relapse to rationally designed
combinations
Lorlatinib I NCT03107988 ALK Recruiting Lorlatinib as a single agent and in
combination with chemotherapy in children
with relapsed/refractory neuroblastoma
Entrectinib I NCT02650401 ALK fusions, Recruiting For recurrent or refractory solid tumors,
TRK & ROS1 with or without TRK, ROS1 or ALK fusions
Ensartinib, Selumetinib II NCT03155620 ALK, activating Recruiting Ensartinib, Selumetinib sulfate and
sulfate & Ulixertinib MAPK pathway Ulixertinib are in a larger panel of
mutations and compounds being investigated in The
MAPK pathway Pediatric MATCH Screening Trial, for
mutations refractory or relapsing pediatric cancers
respectively
inhibitor. However both compounds were not well tolerated in pediatric Phase I and II trials [13-17] . MYCN
status did not improve response and it is unknown whether MYCN is selectively targeted in these tumors.
The way remains open for suggestions of alternative or combination studies. For example, it is predicted
that the combination targeting of Aurora-A and ATR is beneficial in MYCN-driven tumors, as Aurora-A
[9]
inhibitors activates ATR .
Recently, a set of self-regulated master transcription factors known as the “core transcriptional regulatory
circuits” have been described to maintain MYCN-amplified neuroblastoma in a state of pro-growth
[18]
and pro-survival . The identification of the key genes involved in maintaining these tumors will allow
researchers to focus on finding specific vulnerabilities in the pathways that they control. In addition,
indirect approaches to block transcriptional output of MYCN have gained traction with the development
of improved genetic and chemical tools targeting various components of the transcriptional machinery
that chaperone MYC to target promotors and enhancers.