Page 66 - Read Online
P. 66
Tarantino et al. Cancer Drug Resist 2019;2:43-52 I http://dx.doi.org/10.20517/cdr.2018.22 Page 45
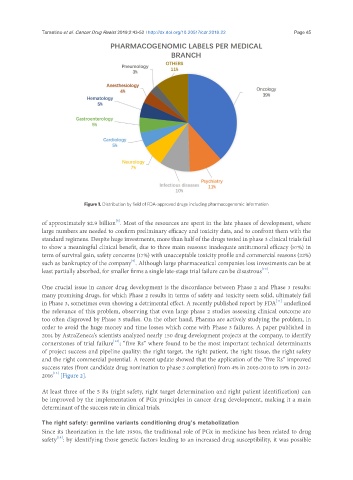
Figure 1. Distribution by field of FDA-approved drugs including pharmacogenomic information
[8]
of approximately $2.9 billion . Most of the resources are spent in the late phases of development, where
large numbers are needed to confirm preliminary efficacy and toxicity data, and to confront them with the
standard regimens. Despite huge investments, more than half of the drugs tested in phase 3 clinical trials fail
to show a meaningful clinical benefit, due to three main reasons: inadequate antitumoral efficacy (57%) in
term of survival gain, safety concerns (17%) with unacceptable toxicity profile and commercial reasons (22%)
such as bankruptcy of the company . Although large pharmaceutical companies loss investments can be at
[9]
[10]
least partially absorbed, for smaller firms a single late-stage trial failure can be disastrous .
One crucial issue in cancer drug development is the discordance between Phase 2 and Phase 3 results:
many promising drugs, for which Phase 2 results in terms of safety and toxicity seem solid, ultimately fail
[11]
in Phase 3, sometimes even showing a detrimental effect. A recently published report by FDA underlined
the relevance of this problem, observing that even large phase 2 studies assessing clinical outcome are
too often disproved by Phase 3 studies. On the other hand, Pharma are actively studying the problem, in
order to avoid the huge money and time losses which come with Phase 3 failures. A paper published in
2014 by AstraZeneca’s scientists analyzed nearly 150 drug development projects at the company, to identify
cornerstones of trial failure ; “five Rs” where found to be the most important technical determinants
[12]
of project success and pipeline quality: the right target, the right patient, the right tissue, the right safety
and the right commercial potential. A recent update showed that the application of the “five Rs” improved
success rates (from candidate drug nomination to phase 3 completion) from 4% in 2005-2010 to 19% in 2012-
2016 [Figure 2].
[13]
At least three of the 5 Rs (right safety, right target determination and right patient identification) can
be improved by the implementation of PGx principles in cancer drug development, making it a main
determinant of the success rate in clinical trials.
The right safety: germline variants conditioning drug’s metabolization
Since its theorization in the late 1950s, the traditional role of PGx in medicine has been related to drug
safety : by identifying those genetic factors leading to an increased drug susceptibility, it was possible
[14]