Page 67 - Read Online
P. 67
Page 166 Genovese et al. Cancer Drug Resist 2018;1:164-80 I http://dx.doi.org/10.20517/cdr.2018.10
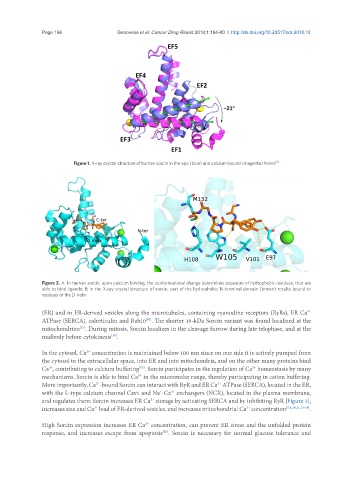
Figure 1. X-ray crystal structure of human sorcin in the apo (blue) and calcium-bound (magenta) forms [5]
Figure 2. A: In human sorcin, upon calcium binding, the conformational change determines exposure of hydrophobic residues, that are
able to bind ligands; B: in the X-ray crystal structure of sorcin, part of the hydrophobic N-terminal domain (brown) results bound to
residues of the D-helix
(ER) and in ER-derived vesicles along the microtubules, containing ryanodine receptors (RyRs), ER Ca 2+
ATPase (SERCA), calreticulin and Rab10 . The shorter 19-kDa Sorcin variant was found localized at the
[10]
mitochondrion . During mitosis, Sorcin localizes in the cleavage furrow during late telophase, and at the
[11]
midbody before cytokinesis .
[10]
In the cytosol, Ca concentration is maintained below 100 nm since on one side it is actively pumped from
2+
the cytosol to the extracellular space, into ER and into mitochondria, and on the other many proteins bind
Ca , contributing to calcium buffering . Sorcin participates in the regulation of Ca homeostasis by many
2+
2+
[12]
2+
mechanisms. Sorcin is able to bind Ca in the micromolar range, thereby participating in cation buffering.
More importantly, Ca -bound Sorcin can interact with RyR and ER Ca ATPase (SERCA), located in the ER,
2+
2+
with the L-type calcium channel Cav1 and Na -Ca exchangers (NCX), located in the plasma membrane,
2+
+
and regulates them: Sorcin increases ER Ca storage by activating SERCA and by inhibiting RyR [Figure 3],
2+
2+
increases size and Ca load of ER-derived vesicles, and increases mitochondrial Ca concentration [7,8,10,11,13-19] .
2+
High Sorcin expression increases ER Ca concentration, can prevent ER stress and the unfolded protein
2+
response, and increases escape from apoptosis . Sorcin is necessary for normal glucose tolerance and
[10]