Page 76 - Read Online
P. 76
Sharma et al. Cancer Drug Resist 2023;6:688-708 https://dx.doi.org/10.20517/cdr.2023.82 Page 692
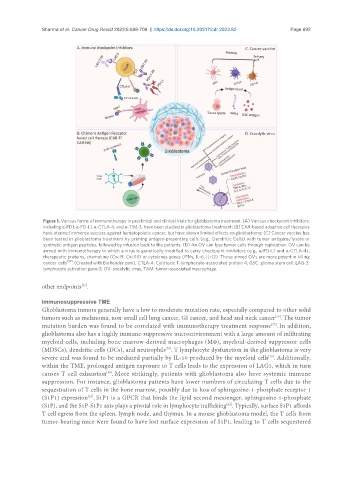
Figure 1. Various forms of immunotherapy in preclinical and clinical trials for glioblastoma treatment. (A) Various checkpoint inhibitors,
including α-PD1, α-PD-L1, α-CTLA-4, and α-TIM-3, have been studied in glioblastoma treatment; (B) CAR-based adoptive cell therapies
have attained immense success against hematopoietic cancer, but have shown limited effects on glioblastoma; (C) Cancer vaccine has
been tested in glioblastoma treatment by priming antigen-presenting cells (e.g., Dendritic Cells) with tumor antigens/lysate or
synthetic antigen peptides, followed by infusion back to the patients; (D) An OV can lyse tumor cells through replication. OV can be
armed with immunotherapy in which a virus is genetically modified to carry checkpoint inhibitors (e.g., α-PD-L1 and α-CTLA-4),
therapeutic proteins, chemokine (Cxcl9, Cxcl10) or cytokines genes (IFNγ, IL-6, IL-12). Those armed OVs are more potent in killing
cancer cells [52] . (Created with BioRender.com). CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; GSC: glioma stem cell; LAG-3:
lymphocyte activation gene-3; OV: oncolytic virus; TAM: tumor-associated macrophage.
other endpoints .
[75]
Immunosuppressive TME
Glioblastoma tumors generally have a low to moderate mutation rate, especially compared to other solid
tumors such as melanoma, non-small cell lung cancer, GI cancer, and head and neck cancer . The tumor
[76]
mutation burden was found to be correlated with immunotherapy treatment response . In addition,
[77]
glioblastoma also has a highly immune-suppressive microenvironment with a large amount of infiltrating
myeloid cells, including bone marrow-derived macrophages (MΦ), myeloid-derived suppressor cells
(MDSCs), dendritic cells (DCs), and neutrophils . T lymphocyte dysfunction in the glioblastoma is very
[78]
severe and was found to be mediated partially by IL-10 produced by the myeloid cells . Additionally,
[79]
within the TME, prolonged antigen exposure to T cells leads to the expression of LAG3, which in turn
causes T cell exhaustion . More strikingly, patients with glioblastoma also have systemic immune
[80]
suppression. For instance, glioblastoma patients have lower numbers of circulating T cells due to the
sequestration of T cells in the bone marrow, possibly due to loss of sphingosine-1-phosphate receptor 1
(S1P1) expression . S1P1 is a GPCR that binds the lipid second messenger, sphingosine-1-phosphate
[81]
(S1P), and the S1P-S1P1 axis plays a pivotal role in lymphocyte trafficking . Typically, surface S1P1 affords
[82]
T cell egress from the spleen, lymph node, and thymus. In a mouse glioblastoma model, the T cells from
tumor-bearing mice were found to have lost surface expression of S1P1, leading to T cells sequestered