Page 8 - Read Online
P. 8
Page 4 of 10 Mazur et al. Rare Dis Orphan Drugs J 2023;2:1 https://dx.doi.org/10.20517/rdodj.2022.12
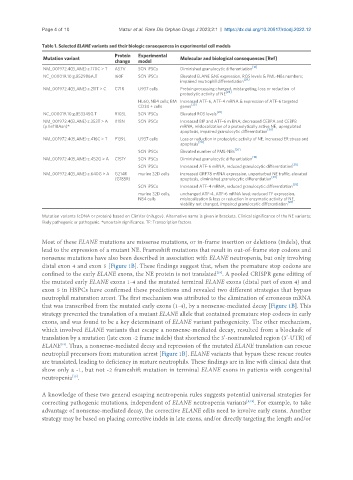
Table 1. Selected ELANE variants and their biologic consequences in experimental cell models
Protein Experimental
Mutation variant Molecular and biological consequences [Ref]
change model
[18]
NM_001972.4(ELANE):c.170C > T A57V SCN iPSCs Diminished granulocytic differentiation
NC_000019.10:g.852986A.T I60F SCN iPSCs Elevated ELANE &NE expression, ROS levels & PML-NBs numbers;
[26]
impaired neutrophil differentiation
NM_001972.4(ELANE):c.211T > C C71R U937 cells Protein processing changed, mistargeting, loss or reduction of
[28]
proteolytic activity of NE
HL60, NB4 cells; BM Increased ATF-6, ATF-4 mRNA & expression of ATF-6 targeted
CD34 + cells genes [25]
[26]
NC_000019.10:g.853345G.T R103L SCN iPSCs Elevated ROS levels
NM_001972.4(ELANE):c.353T > A I118N SCN iPSCs Increased BIP and ATF-6 m RNA; decreased CEBPA and CEBPB
(p.Ile118Asn)* mRNA, mislocalization of a proteolytically active NE, upregulated
apoptosis, impaired granulocytic differentiation [30]
NM_001972.4(ELANE):c.416C > T P139L U937 cells Loss or reduction in proteolytic activity of NE, increased ER stress and
apoptosis [28]
[26]
SCN iPSCs Elevated number of PML-NBs
NM_001972.4(ELANE):c.452G > A C151Y SCN iPSCs Diminished granulocytic differentiation [18]
[38]
SCN iPSCs Increased ATF-6 mRNA, reduced granulocytic differentiation
NM_001972.4(ELANE):c.640G > A G214R murine 32D cells increased GRP78 mRNA expression, unperturbed NE traffic, elevated
[39]
(G185R) apoptosis, diminished granulocytic differentiation
[38]
SCN iPSCs Increased ATF-4 mRNA, reduced granulocytic differentiation
murine 32D cells, unchanged ATF-4, ATF-6 mRNA level; reduced TF expression,
NB4 cells mislocalization & loss or reduction in enzymatic activity of NE,
viability not changed, impaired granulocytic differentiation [22]
Mutation variants (cDNA or protein) based on ClinVar (nih.gov). Alternative name is given in brackets. Clinical significance of the NE variants:
likely pathogenic or pathogenic. *uncertain significance. TF: Transcription factors.
Most of these ELANE mutations are missense mutations, or in-frame insertion or deletions (indels), that
lead to the expression of a mutant NE. Frameshift mutations that result in out-of-frame stop codons and
nonsense mutations have also been described in association with ELANE neutropenia, but only involving
distal exon 4 and exon 5 [Figure 1B]. These findings suggest that, when the premature stop codons are
confined to the early ELANE exons, the NE protein is not translated . A pooled CRISPR gene editing of
[19]
the mutated early ELANE exons 1-4 and the mutated terminal ELANE exons (distal part of exon 4) and
exon 5 in HSPCs have confirmed these predictions and revealed two different strategies that bypass
neutrophil maturation arrest. The first mechanism was attributed to the elimination of erroneous mRNA
that was transcribed from the mutated early exons (1-4), by a nonsense-mediated decay [Figure 1B]. This
strategy prevented the translation of a mutant ELANE allele that contained premature stop codons in early
exons, and was found to be a key determinant of ELANE variant pathogenicity. The other mechanism,
which involved ELANE variants that escape a nonsense-mediated decay, resulted from a blockade of
translation by a mutation (late exon -2 frame indels) that shortened the 3’-nontranslated region (3’-UTR) of
ELANE . Thus, a nonsense-mediated decay and repression of the mutated ELANE translation can rescue
[19]
neutrophil precursors from maturation arrest [Figure 1B]. ELANE variants that bypass these rescue routes
are translated, leading to deficiency in mature neutrophils. These findings are in line with clinical data that
show only a -1, but not -2 frameshift mutation in terminal ELANE exons in patients with congenital
neutropenia .
[19]
A knowledge of these two general escaping neutropenia rules suggests potential universal strategies for
correcting pathogenic mutations, independent of ELANE neutropenia variants [8,19] . For example, to take
advantage of nonsense-mediated decay, the corrective ELANE edits need to involve early exons. Another
strategy may be based on placing corrective indels in late exons, and/or directly targeting the length and/or