Page 22 - Read Online
P. 22
Page 8 of 10 Chu et al. Rare Dis Orphan Drugs J 2023;2:2 https://dx.doi.org/10.20517/rdodj.2022.25
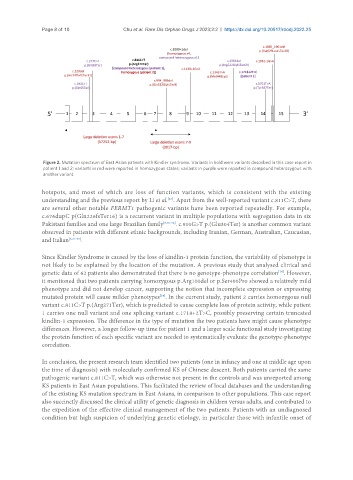
Figure 2. Mutation spectrum of East Asian patients with Kindler syndrome. Variants in bold were variants described in this case report in
patient 1 and 2; variants in red were reported in homozygous states; variants in purple were reported in compound heterozygous with
another variant.
hotspots, and most of which are loss of function variants, which is consistent with the existing
understanding and the previous report by Li et al. . Apart from the well-reported variant c.811C>T, there
[23]
are several other notable FERMT1 pathogenic variants have been reported repeatedly. For example,
c.676dupC p(Gln226fsTer16) is a recurrent variant in multiple populations with segregation data in six
Pakistani families and one large Brazilian family [6,30-32] . c.910G>T p.(Glu304Ter) is another common variant
observed in patients with different ethnic backgrounds, including Iranian, German, Australian, Caucasian,
and Italian [6,32-35] .
Since Kindler Syndrome is caused by the loss of kindlin-1 protein function, the variability of phenotype is
not likely to be explained by the location of the mutation. A previous study that analysed clinical and
[14]
genetic data of 62 patients also demonstrated that there is no genotype-phenotype correlation . However,
it mentioned that two patients carrying homozygous p.Arg100del or p.Ser400Pro showed a relatively mild
phenotype and did not develop cancer, supporting the notion that incomplete expression or expressing
mutated protein will cause milder phenotypes . In the current study, patient 2 carries homozygous null
[14]
variant c.811C>T p.(Arg271Ter), which is predicted to cause complete loss of protein activity, while patient
1 carries one null variant and one splicing variant c.1718+2T>C, possibly preserving certain truncated
kindlin-1 expression. The difference in the type of mutation the two patients have might cause phenotype
differences. However, a longer follow-up time for patient 1 and a larger scale functional study investigating
the protein function of each specific variant are needed to systematically evaluate the genotype-phenotype
correlation.
In conclusion, the present research team identified two patients (one in infancy and one at middle age upon
the time of diagnosis) with molecularly confirmed KS of Chinese descent. Both patients carried the same
pathogenic variant c.811C>T, which was otherwise not present in the controls and was unreported among
KS patients in East Asian populations. This facilitated the review of local databases and the understanding
of the existing KS mutation spectrum in East Asians, in comparison to other populations. This case report
also succinctly discussed the clinical utility of genetic diagnosis in children versus adults, and contributed to
the expedition of the effective clinical management of the two patients. Patients with an undiagnosed
condition but high suspicion of underlying genetic etiology, in particular those with infantile onset of