Page 214 - Read Online
P. 214
Cheng et al. Vessel Plus 22020;4:17 I http://dx.doi.org/10.20517/2574-1209.2020.08 Page 7 of 15
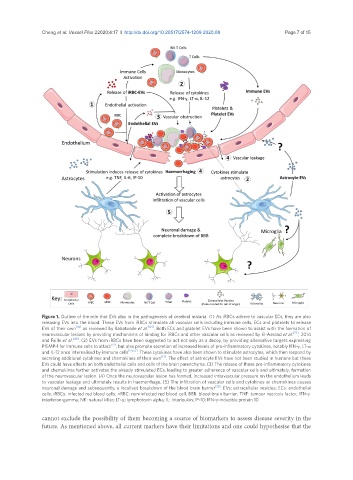
Figure 1. Outline of the role that EVs play in the pathogenesis of cerebral malaria. (1) As iRBCs adhere to vascular ECs, they are also
releasing EVs into the blood. These EVs from iRBCs stimulate all vascular cells including immune cells, ECs and platelets to release
EVs of their own [83] as reviewed by Babatunde et al. [84] . Both ECs and platelet EVs have been shown to assist with the formation of
neurovascular lesions by providing mechanisms of binding for iRBCs and other vascular cells as reviewed by El-Assaad et al. [77] , 2014
and Faille et al. [49] . (2) EVs from iRBCs have been suggested to act not only as a decoy, by providing alternative targets expressing
PfEMP-1 for immune cells to attack [51] , but also promote secretion of increased levels of pro-inflammatory cytokines, notably IFN-γ, LT-α
and IL-12 once internalised by immune cells [54,55] . These cytokines have also been shown to stimulate astrocytes, which then respond by
secreting additional cytokines and chemokines of their own [13] . The effect of astrocyte EVs have not been studied in humans but these
EVs could have effects on both endothelial cells and cells of the brain parenchyma. (3) The release of these pro-inflammatory cytokines
and chemokines further activates the already stimulated ECs, leading to greater adherence of vascular cells and ultimately, formation
of the neurovascular lesion. (4) Once the neurovascular lesion has formed, increased intravascular pressure on the endothelium leads
to vascular leakage and ultimately results in haemorrhage. (5) The infiltration of vascular cells and cytokines or chemokines causes
neuronal damage and subsequently, a localised breakdown of the blood brain barrier [32] . EVs: extracellular vesicles; ECs: endothelial
cells; iRBCs: infected red blood cells; nRBC: non-infected red blood cell; BBB: blood-brain barrier; TNF: tumour necrosis factor; IFN-γ:
interferon-gamma; NK: natural killer; LT-α: lymphotoxin alpha; IL: Interleukin; IP-10: IFN-γ-inducible protein 10
cannot exclude the possibility of them becoming a source of biomarkers to assess disease severity in the
future. As mentioned above, all current markers have their limitations and one could hypothesise that the