Page 31 - Read Online
P. 31
Shah et al. Vessel Plus 2021;5:53 https://dx.doi.org/10.20517/2574-1209.2021.76 Page 3 of 11
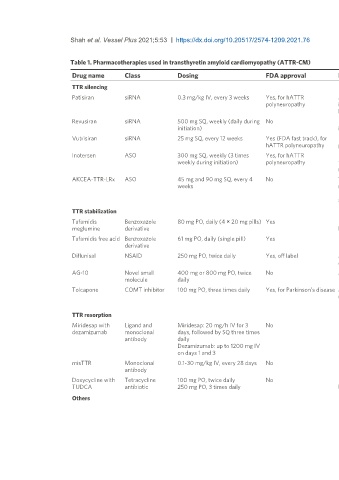
Table 1. Pharmacotherapies used in transthyretin amyloid cardiomyopathy (ATTR-CM)
Drug name Class Dosing FDA approval Key points Notable adverse effects
TTR silencing
[13]
Patisiran siRNA 0.3 mg/kg IV, every 3 weeks Yes, for hATTR Mortality benefit , improvement in neuropathy Vitamin A deficiency, infusion-related
[13]
polyneuropathy impairment scores , potential reduction in cardiac reactions
[14]
hospitalizations and deaths
Revusiran siRNA 500 mg SQ, weekly (daily during No Phase III trial terminated early due to increased mortality Sudden cardiac death, congestive cardiac
[15]
initiation) in treatment arm failure
Vutrisiran siRNA 25 mg SQ, every 12 weeks Yes (FDA fast track), for Phase 3 trial recently completed, but data not yet Not reported
[16]
hATTR polyneuropathy presented or published
[18]
Inotersen ASO 300 mg SQ, weekly (3 times Yes, for hATTR Improvement in neuropathy impairment scores , trend Thrombocytopenia, glomerulonephritis,
[18]
weekly during initiation) polyneuropathy towards mortality benefit vitamin A deficiency, infusion-related reactions
requires weekly CBC and biweekly BMP and UA
AKCEA-TTR-LRx ASO 45 mg and 90 mg SQ, every 4 No Targeted delivery of inostersen-like compound to liver; Headaches, liver enzyme abnormalities,
weeks may reduce safety concerns increase in blood creatine phosphokinase, flu-
Phase 3 clinical trials underway (CARDIO-TTRansform like illness
and NEURO-TTRansform)
TTR stabilization
Tafamidis Benzoxazole 80 mg PO, daily (4 × 20 mg pills) Yes Reduction in all-cause mortality and cardiovascular Allergic reactions, GI distress, headache
[20]
meglumine derivative hospitalization
[21]
Tafamidis free acid Benzoxazole 61 mg PO, daily (single pill) Yes Lower pill burden than tafamidis meglumine Allergic reactions, GI distress, headache
[21]
derivative Potentially less GI distress
[23]
Diflunisal NSAID 250 mg PO, twice daily Yes, off label May have comparable mortality benefit as tafamidis , Thrombocytopenia, renal dysfunction, fluid
avoid concomitant NSAID use, administer with PPI retention
[24]
AG-10 Novel small 400 mg or 800 mg PO, twice No More robust response in ATTRm than ATTRwt , Phase Atrial fibrillation, congestive heart failure,
molecule daily III trial underway cellulitis, and dyspnea
[25]
Tolcapone COMT inhibitor 100 mg PO, three times daily Yes, for Parkinson’s disease In vitro data only currently available , prospectively trial FDA black box warning for hepatotoxicity
underway Dyskinesia, GI distress, sleep disturbance
TTR resorption
[26]
Miridesap with Ligand and Miridesap: 20 mg/h IV for 3 No Phase II study terminated early Serious rashes, others not reported
dezamizumab monoclonal days, followed by SQ three times
antibody daily
Dezamizumab: up to 1200 mg IV
on days 1 and 3
[28]
misTTR Monoclonal 0.1-30 mg/kg IV, every 28 days No Phase I study recently completed Fall, anemia, URI, back pain, GI distress,
antibody insomnia
[29]
Doxycycline with Tetracycline 100 mg PO, twice daily No Phase II study showed reduction disease progression , Rash, GI distress
TUDCA antibiotic 250 mg PO, 3 times daily but high rate of serious adverse effects in follow-up study
Others