Page 18 - Read Online
P. 18
Carciotto et al. Vessel Plus 2024;8:33 https://dx.doi.org/10.20517/2574-1209.2024.01 Page 9 of 13
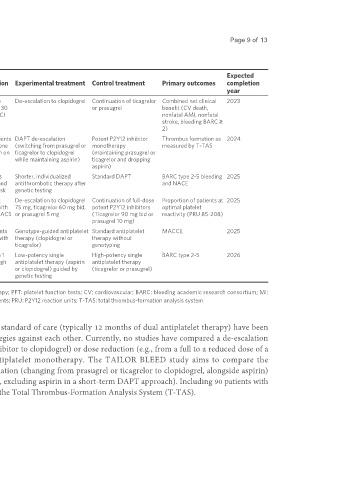
Table 2. Future randomized controlled trials testing a de-escalation strategy
Expected
Estimated
Study title NCT number Primary objective Target population Experimental treatment Control treatment Primary outcomes completion
enrollment
year
VERONICA NCT04654052 634 Optimize platelet inhibition therapy in ACS patients with De-escalation to clopidogrel Continuation of ticagrelor Combined net clinical 2023
ACS patients using PFT VerifyNow PRU ≤ 30 or prasugrel benefit (CV death,
at 30 days after PCI nonfatal AMI, nonfatal
stroke, bleeding BARC ≥
2)
TAILOR NCT05681702 90 Compare the pharmacodynamic ACS and CCS patients DAPT de-escalation Potent P2Y12 inhibitor Thrombus formation as 2024
BLEED effects of two bleeding reduction who have undergone (switching from prasugrel or monotherapy measured by T-TAS
strategies in patients undergoing PCI PCI and have been on ticagrelor to clopidogrel (maintaining prasugrel or
DAPT while maintaining aspirin) ticagrelor and dropping
aspirin)
Dan-DAPT NCT05262803 2,808 Evaluate a reduced antithrombotic Type 1 MI patients Shorter, individualized Standard DAPT BARC type 2-5 bleeding 2025
strategy in high bleeding risk patients treated with PCI and antithrombotic therapy after and NACE
post-MI at high bleeding risk genetic testing
DESC-HBR NCT05277987 200 Assess the impact of de-escalating High bleeding risk De-escalation to clopidogrel Continuation of full-dose Proportion of patients at 2025
P2Y12 inhibitor therapy in high patients treated with 75 mg, ticagrelor 60 mg bid, potent P2Y12 inhibitors optimal platelet
bleeding risk patients post-ACS PCI due to recent ACS or prasugrel 5 mg (Ticagrelor 90 mg bid or reactivity (PRU 85-208)
prasugrel 10 mg)
GUARANTEE NCT05277987 4,009 Evaluate effectiveness and security of ACS or CCS patients Genotype-guided antiplatelet Standard antiplatelet MACCE 2025
CYP2C19 genotype-guided antiplatelet treated with PCI with therapy (clopidogrel or therapy without
therapy DES ticagrelor) genotyping
ADEN NCT05577988 2,468 Compare early de-escalation to low- Patients with type 1 Low-potency single High-potency single BARC type 2-5 2026
potency single antiplatelet therapy MI classified as high antiplatelet therapy (aspirin antiplatelet therapy
guided by genetics vs. high-potency bleeding risk or clopidogrel) guided by (ticagrelor or prasugrel)
therapy in high bleeding risk patients genetic testing
ACS: Acute coronary syndrome; PCI: percutaneous coronary interventions; DAPT: dual antiplatelet therapy; PFT: platelet function tests; CV: cardiovascular; BARC: bleeding academic research consortium; MI:
myocardial infarction; ST: stent thrombosis; MACCE: major adverse cardiovascular and cerebrovascular events; PRU: P2Y12 reaction units; T-TAS: total thrombus-formation analysis system.
comparisons between P2Y12i switching, dose reduction, or interruption and standard of care (typically 12 months of dual antiplatelet therapy) have been
conducted, there are no randomized studies that directly compare these strategies against each other. Currently, no studies have compared a de-escalation
strategy based on switching antiplatelet therapy (e.g., from a potent P2Y12 inhibitor to clopidogrel) or dose reduction (e.g., from a full to a reduced dose of a
potent P2Y12 inhibitor) against short-term DAPT followed by single antiplatelet monotherapy. The TAILOR BLEED study aims to compare the
pharmacodynamic effects of two bleeding reduction strategies: DAPT de-escalation (changing from prasugrel or ticagrelor to clopidogrel, alongside aspirin)
versus potent P2Y12 inhibitor monotherapy (continuing prasugrel or ticagrelor, excluding aspirin in a short-term DAPT approach). Including 90 patients with
both CCS and ACS, the primary outcome is thrombus formation, measured by the Total Thrombus-Formation Analysis System (T-TAS).