Page 124 - Read Online
P. 124
Gomes et al. Vessel Plus 2023;7:24 https://dx.doi.org/10.20517/2574-1209.2023.60 Page 3 of 16
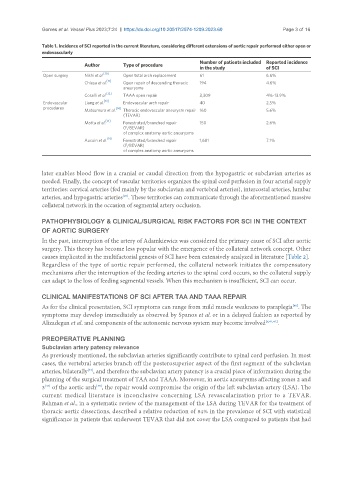
Table 1. Incidence of SCI reported in the current literature, considering different extensions of aortic repair performed either open or
endovascularly
Number of patients included Reported incidence
Author Type of procedure
in the study of SCI
[10]
Open surgery Nishi et al. Open total arch replacement 61 6.6%
Chiesa et al. [11] Open repair of descending thoracic 194 4.6%
aneurysms
[12]
Coselli et al. TAAA open repair 3,309 4%-13.9%
[13]
Endovascular Liang et al. Endovascular arch repair 40 2.5%
procedures [14]
Matsumura et al. Thoracic endovascular aneurysm repair 160 5.6%
(TEVAR)
[15]
Motta et al. Fenestrated/branched repair 150 2.6%
(F/BEVAR)
of complex anatomy aortic aneurysms
Aucoin et al. [16] Fenestrated/branched repair 1,681 7.1%
(F/BEVAR)
of complex anatomy aortic aneurysms
later enables blood flow in a cranial or caudal direction from the hypogastric or subclavian arteries as
needed. Finally, the concept of vascular territories organizes the spinal cord perfusion in four arterial supply
territories: cervical arteries (fed mainly by the subclavian and vertebral arteries), intercostal arteries, lumbar
[25]
arteries, and hypogastric arteries . These territories can communicate through the aforementioned massive
collateral network in the occasion of segmental artery occlusion.
PATHOPHYSIOLOGY & CLINICAL/SURGICAL RISK FACTORS FOR SCI IN THE CONTEXT
OF AORTIC SURGERY
In the past, interruption of the artery of Adamkiewicz was considered the primary cause of SCI after aortic
surgery. This theory has become less popular with the emergence of the collateral network concept. Other
causes implicated in the multifactorial genesis of SCI have been extensively analyzed in literature [Table 2].
Regardless of the type of aortic repair performed, the collateral network initiates the compensatory
mechanisms after the interruption of the feeding arteries to the spinal cord occurs, so the collateral supply
can adapt to the loss of feeding segmental vessels. When this mechanism is insufficient, SCI can occur.
CLINICAL MANIFESTATIONS OF SCI AFTER TAA AND TAAA REPAIR
As for the clinical presentation, SCI symptoms can range from mild muscle weakness to paraplegia . The
[40]
symptoms may develop immediately as observed by Spanos et al. or in a delayed fashion as reported by
Alizadegan et al. and components of the autonomic nervous system may become involved [6,41,42] .
PREOPERATIVE PLANNING
Subclavian artery patency relevance
As previously mentioned, the subclavian arteries significantly contribute to spinal cord perfusion. In most
cases, the vertebral arteries branch off the posterosuperior aspect of the first segment of the subclavian
arteries, bilaterally , and therefore the subclavian artery patency is a crucial piece of information during the
[43]
planning of the surgical treatment of TAA and TAAA. Moreover, in aortic aneurysms affecting zones 2 and
3 of the aortic arch , the repair would compromise the origin of the left subclavian artery (LSA). The
[44]
[45]
current medical literature is inconclusive concerning LSA revascularization prior to a TEVAR.
Rehman et al., in a systematic review of the management of the LSA during TEVAR for the treatment of
thoracic aortic dissections, described a relative reduction of 84% in the prevalence of SCI with statistical
significance in patients that underwent TEVAR that did not cover the LSA compared to patients that had