Page 40 - Read Online
P. 40
Page 4 of 12 Politei et al. Rare Dis Orphan Drugs J 2024;3:10 https://dx.doi.org/10.20517/rdodj.2023.46
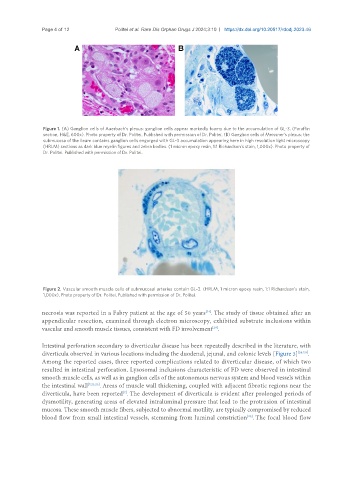
Figure 1. (A) Ganglion cells of Auerbach’s plexus: ganglion cells appear markedly foamy due to the accumulation of GL-3. (Paraffin
section, H&E, 600x). Photo property of Dr. Politei. Published with permission of Dr. Politei. (B) Ganglion cells of Meissner’s plexus: the
submucosa of the ileum contains ganglion cells engorged with GL-3 accumulation appearing here in high resolution light microscopy
(HRLM) sections as dark blue myelin figures and zebra bodies. (1 micron epoxy resin, 1:1 Richardson’s stain, 1,000x). Photo property of
Dr. Politei. Published with permission of Dr. Politei.
Figure 2. Vascular smooth muscle cells of submucosal arteries contain GL-3. (HRLM, 1 micron epoxy resin, 1:1 Richardson’s stain,
1,000x). Photo property of Dr. Politei. Published with permission of Dr. Politei.
[24]
necrosis was reported in a Fabry patient at the age of 50 years . The study of tissue obtained after an
appendicular resection, examined through electron microscopy, exhibited substrate inclusions within
vascular and smooth muscle tissues, consistent with FD involvement .
[28]
Intestinal perforation secondary to diverticular disease has been repeatedly described in the literature, with
diverticula observed in various locations including the duodenal, jejunal, and colonic levels [Figure 3] [24,7,8] .
Among the reported cases, three reported complications related to diverticular disease, of which two
resulted in intestinal perforation. Lysosomal inclusions characteristic of FD were observed in intestinal
smooth muscle cells, as well as in ganglion cells of the autonomous nervous system and blood vessels within
the intestinal wall [7,21,23] . Areas of muscle wall thickening, coupled with adjacent fibrotic regions near the
[7]
diverticula, have been reported . The development of diverticula is evident after prolonged periods of
dysmotility, generating areas of elevated intraluminal pressure that lead to the protrusion of intestinal
mucosa. These smooth muscle fibers, subjected to abnormal motility, are typically compromised by reduced
blood flow from small intestinal vessels, stemming from luminal constriction . The focal blood flow
[29]