Page 110 - Read Online
P. 110
Page 22 of 34 West et al. Rare Dis Orphan Drugs J 2024;3:22 https://dx.doi.org/10.20517/rdodj.2023.61
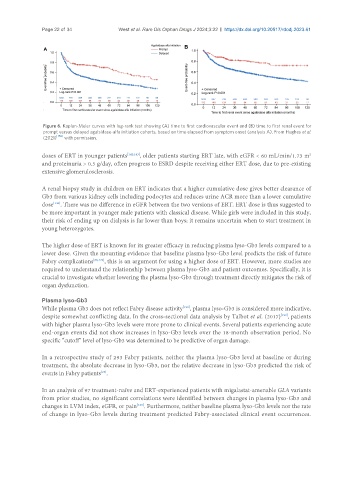
Figure 6. Kaplan-Meier curves with log-rank test showing (A) time to first cardiovascular event and (B) time to first renal event for
prompt versus delayed agalsidase-alfa initiation cohorts, based on time elapsed from symptom onset (analysis A). From Hughes et al.
(2021) [156] with permission.
doses of ERT in younger patients [142,143] , older patients starting ERT late, with eGFR < 60 mL/min/1.73 m
2
and proteinuria > 0.5 g/day, often progress to ESRD despite receiving either ERT dose, due to pre-existing
extensive glomerulosclerosis.
A renal biopsy study in children on ERT indicates that a higher cumulative dose gives better clearance of
Gb3 from various kidney cells including podocytes and reduces urine ACR more than a lower cumulative
dose . There was no difference in eGFR between the two versions of ERT. ERT dose is thus suggested to
[146]
be more important in younger male patients with classical disease. While girls were included in this study,
their risk of ending up on dialysis is far lower than boys; it remains uncertain when to start treatment in
young heterozygotes.
The higher dose of ERT is known for its greater efficacy in reducing plasma lyso-Gb3 levels compared to a
lower dose. Given the mounting evidence that baseline plasma lyso-Gb3 level predicts the risk of future
Fabry complications [68,120] , this is an argument for using a higher dose of ERT. However, more studies are
required to understand the relationship between plasma lyso-Gb3 and patient outcomes. Specifically, it is
crucial to investigate whether lowering the plasma lyso-Gb3 through treatment directly mitigates the risk of
organ dysfunction.
Plasma lyso-Gb3
While plasma Gb3 does not reflect Fabry disease activity , plasma lyso-Gb3 is considered more indicative,
[158]
despite somewhat conflicting data. In the cross-sectional data analysis by Talbot et al. (2017) , patients
[159]
with higher plasma lyso-Gb3 levels were more prone to clinical events. Several patients experiencing acute
end-organ events did not show increases in lyso-Gb3 levels over the 18-month observation period. No
specific “cutoff” level of lyso-Gb3 was determined to be predictive of organ damage.
In a retrospective study of 293 Fabry patients, neither the plasma lyso-Gb3 level at baseline or during
treatment, the absolute decrease in lyso-Gb3, nor the relative decrease in lyso-Gb3 predicted the risk of
[29]
events in Fabry patients .
In an analysis of 97 treatment-naïve and ERT-experienced patients with migalastat-amenable GLA variants
from prior studies, no significant correlations were identified between changes in plasma lyso-Gb3 and
changes in LVM index, eGFR, or pain . Furthermore, neither baseline plasma lyso-Gb3 levels nor the rate
[160]
of change in lyso-Gb3 levels during treatment predicted Fabry-associated clinical event occurrences.