Page 44 - Read Online
P. 44
Page 4 of 9 Jain et al. Rare Dis Orphan Drugs J 2024;3:8 https://dx.doi.org/10.20517/rdodj.2023.42
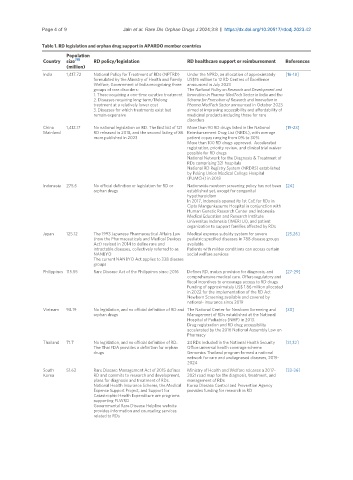
Table 1. RD legislation and orphan drug support in APARDO member countries
Population
Country size [15] RD policy/legislation RD healthcare support or reimbursement References
(million)
India 1,417.72 National Policy for Treatment of RDs (NPTRD) Under the NPRD, an allocation of approximately [16-18]
formulated by the Ministry of Health and Family US$15 million to 12 RD Centres of Excellence
Welfare, Government of India recognizing three announced in July 2023
groups of rare disorders: The National Policy on Research and Development and
1. Those requiring a one-time curative treatment Innovation in Pharma-MedTech Sector in India and the
2. Diseases requiring long-term/lifelong Scheme for Promotion of Research and Innovation in
treatment at a relatively lower cost Pharma MedTech Sector announced in October 2023
3. Diseases for which treatments exist but aimed at improving accessibility and affordability of
remain expensive medicinal products including those for rare
disorders
China 1,412.17 No national legislation on RD. The first list of 121 More than 90 RD drugs listed in the National [19-23]
Mainland RD released in 2018, and the second listing of 86 Reimbursement Drug List (NRDL), with average
more published in 2023 patient copay ranging from 0% to 30%
More than 100 RD drugs approved. Accelerated
registration, priority review, and clinical trial waiver
possible for RD drugs
National Network for the Diagnosis & Treatment of
RDs comprising 321 hospitals
National RD Registry System (NRDRS) established
by Peking Union Medical College Hospital
(PUMCH) in 2018
Indonesia 275.5 No official definition or legislation for RD or Nationwide newborn screening policy has not been [24]
orphan drugs established yet, except for congenital
hypothyroidism
In 2017, Indonesia opened its 1st CoE for RDs in
Cipto Mangunkusumo Hospital in conjunction with
Human Genetic Research Center and Indonesia
Medical Education and Research Institute
Universitas Indonesia (IMERI UI), and patient
organization to support families affected by RDs
Japan 125.12 The 1993 Japanese Pharmaceutical Affairs Law Medical expense subsidy system for severe [25,26]
(now the Pharmaceuticals and Medical Devices pediatric specified diseases in 788 disease groups
Act) revised in 2014 to define rare and available
intractable diseases, collectively referred to as Patients with milder conditions can access certain
NANBYO social welfare services
The current NANBYO Act applies to 338 disease
groups
Philippines 115.55 Rare Disease Act of the Philippines since 2016 Defines RD, makes provision for diagnosis, and [27-29]
comprehensive medical care. Offers regulatory and
fiscal incentives to encourage access to RD drugs
Funding of approximately US$ 1.86 million allocated
in 2022 for the implementation of the RD Act
Newborn Screening available and covered by
national- insurance since 2019
Vietnam 98.19 No legislation, and no official definition of RD and The National Center for Newborn Screening and [30]
orphan drugs Management of RDs established at the National
Hospital of Pediatrics (NHP) in 2013.
Drug registration and RD drug accessibility
accelerated by the 2016 National Assembly Law on
Pharmacy
Thailand 71.7 No legislation, and no official definition of RD. 24 RDs included in the National Health Security [31,32]
The Thai FDA provides a definition for orphan Office universal health coverage scheme
drugs Genomics Thailand program formed a national
network for rare and undiagnosed diseases, 2019-
2024
South 51.63 Rare Disease Management Act of 2015 defines Ministry of Health and Welfare releases a 2017- [33-36]
Korea RD and commits to research and development, 2021 road map for the diagnosis, treatment, and
plans for diagnosis and treatment of RDs. management of RDs
National Health Insurance Scheme, the Medical Korea Disease Control and Prevention Agency
Expense Support Project, and Support for provides funding for research in RD
Catastrophic Health Expenditure are programs
supporting PLWRD
Governmental Rare Disease Helpline website
provides information and counseling services
related to RDs