Page 21 - Read Online
P. 21
Mimouni et al. Rare Dis Orphan Drugs J 2024;3:17 https://dx.doi.org/10.20517/rdodj.2024.06 Page 5 of 10
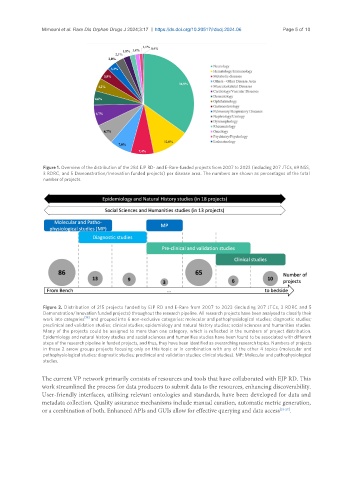
Figure 1. Overview of the distribution of the 284 EJP RD- and E-Rare-funded projects from 2007 to 2023 (including 207 JTCs, 69 NSS,
3 RDRC, and 5 Demonstration/Innovation funded projects) per disease area. The numbers are shown as percentages of the total
number of projects.
Figure 2. Distribution of 215 projects funded by EJP RD and E-Rare from 2007 to 2023 (including 207 JTCs, 3 RDRC and 5
Demonstration/Innovation funded projects) throughout the research pipeline. All research projects have been analysed to classify their
work into categories [19] and grouped into 6 non-exclusive categories: molecular and pathophysiological studies; diagnostic studies;
preclinical and validation studies; clinical studies; epidemiology and natural history studies; social sciences and humanities studies.
Many of the projects could be assigned to more than one category, which is reflected in the numbers of project distribution.
Epidemiology and natural history studies and social sciences and humanities studies have been found to be associated with different
steps of the research pipeline in funded projects, and thus, they have been identified as overarching research topics. Numbers of projects
in those 2 arrow groups projects focusing only on this topic or in combination with any of the other 4 topics (molecular and
pathophysiological studies; diagnostic studies; preclinical and validation studies; clinical studies). MP: Molecular and pathophysiological
studies.
The current VP network primarily consists of resources and tools that have collaborated with EJP RD. This
work streamlined the process for data producers to submit data to the resources, enhancing discoverability.
User-friendly interfaces, utilising relevant ontologies and standards, have been developed for data and
metadata collection. Quality assurance mechanisms include manual curation, automatic metric generation,
or a combination of both. Enhanced APIs and GUIs allow for effective querying and data access [23-27] .